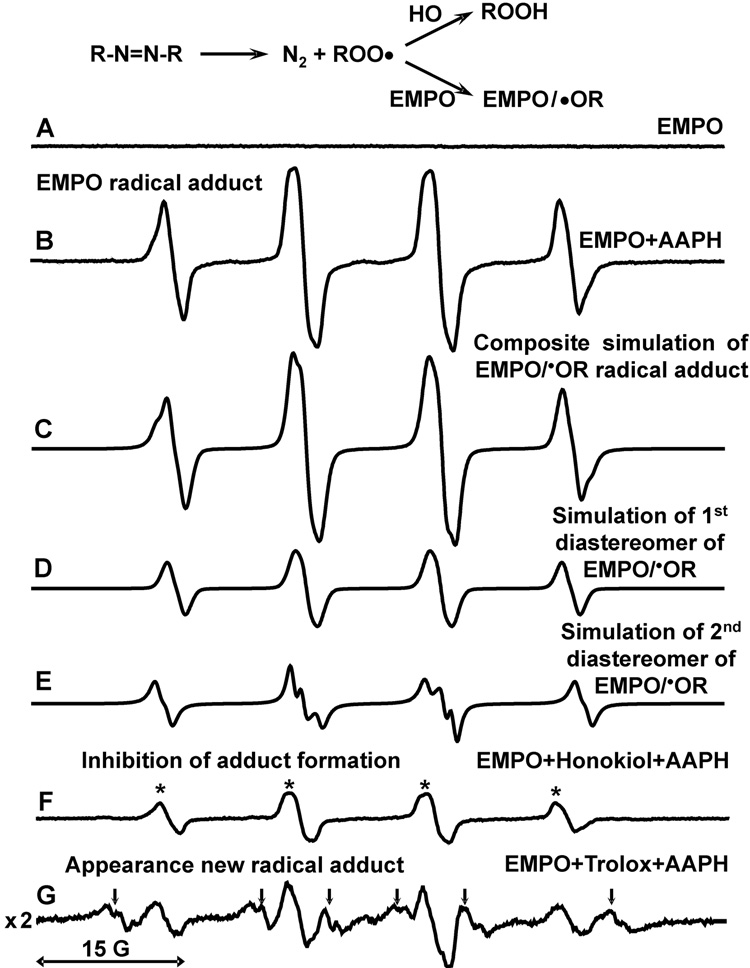
Figure 5.
Scavenging of peroxyl radicals by honokiol. (A) ESR specrum of 60 mM EMPO; (B) specrum of EMPO plus 10 mM AAPH; (C) Composite computer simulation of (B); (D) First component of composite simulation with hyperfine coupling constants aN =13.6 G, aHβ=12.7 G, aHγ=0.9 G; (E) Second component of composite simulation with hyperfine coupling constants aN =13.6 G, aHβ=15.2 G, aHγ=0.9 G; (F) Addition of honokiol (0.2 mM) inhibited formation of the EMPO radical adduct due to scavenging of AAPH derived peroxyl radical by honokiol. (G) Addition of trolox (0.2mM) led to 6-fold decrease of EMPO/•OR amplitude and appearance of new two trolox derived carbon-centered radical adducts indicated by arrows (hyperfine coupling constants aN =14.6 G, aHβ=23.1 G and aN =14.5 G, aHβ=20.6 G). ESR settings were as described in Materials and Methods.