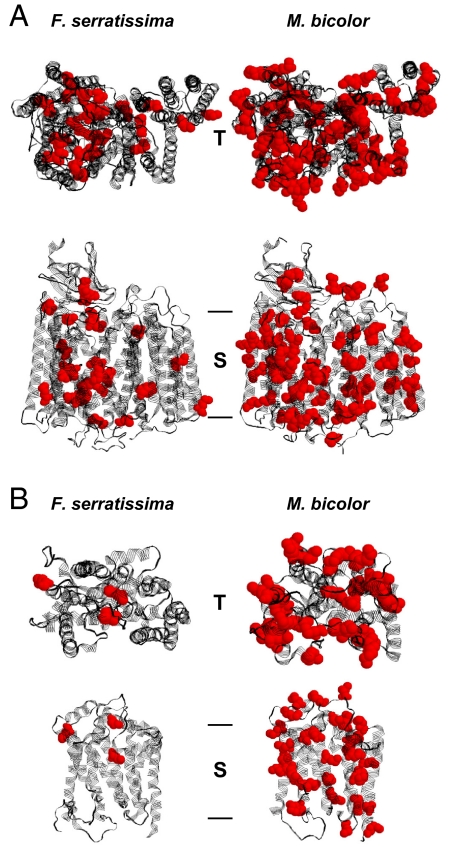
Fig. 2.
Structural models of mitochondrially encoded respiratory chain proteins from the feather star Florometra serratissima and the stingless bee Melipona bicolor. (A) COX core, consisting of COX subunits I, II, and III. COX core is the central part of respiratory chain complex IV. (B) Cytochrome b, the mitochondrially encoded core peptide of ubiquinone-cytochrome c oxidoreductase (respiratory chain complex III). The top view representations (T) approximate the perspective from the mitochondrial intermembrane space. The side view representations (S) show the identical structures as beheld from within the inner mitochondrial membrane, placing the intermembrane space on top. Methionine residues are shown in red. Bars indicate the approximate membrane boundaries. A distinct accumulation of methionine can be discerned in the insect enzymes. The displayed structures correspond to the following methionine contents: COX core: 2.9% in F. serratissima, 7.8% in M. bicolor; cytochrome b: 1.1% in F. serratissima, 9.5% in M. bicolor. Mitochondrially encoded NADH dehydrogenase subunits of M. bicolor exhibited an even more pronounced methionine accumulation, reaching approximately double the level (13.5%) of the displayed COX core structure.