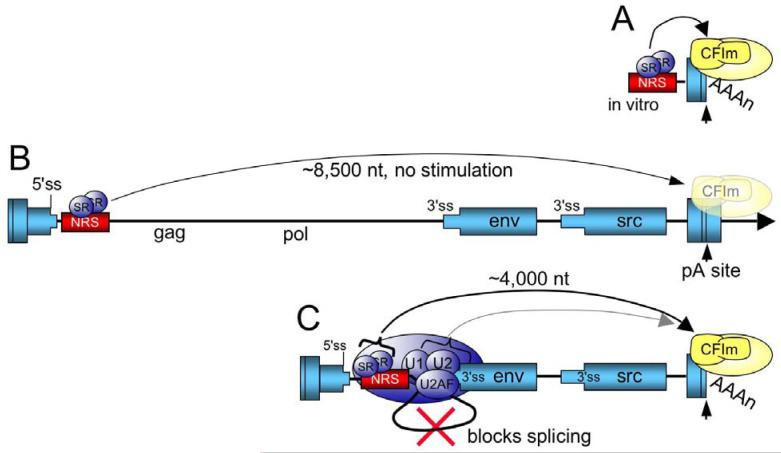
Figure 5.
Model for SR protein stimulation of RSV polyadenylation. A) For in vitro substrates, the NRS is in close proximity to the poly(A) site where SR proteins stimulate polyadenylation, perhaps through an interaction the RS domain with a similar domain within CFIm. CFIm and the polyadenylation machinery are in yellow. B) In the viral context where the NRS-3′ss complex cannot form, SR proteins associated with the NRS are too far away from the poly(A) site to stimulate polyadenylation (washed out poly(A) factors) and read-through transcripts increase (arrow). The RSV ‘exons’ are represented as blue boxes and the intron as a line. C) SR proteins promote NRS complex assembly with the env 3′ ss, which blocks splicing. The NRS-3′ss complex repositions the SR proteins closer to the poly(A) site where they promote poly(A) complex formation (arrow), perhaps through an interaction with CFIm. It is also possible that additional NRS complex factors contribute to poly(A) efficiency through conventional coupling interactions (light shaded arrow).