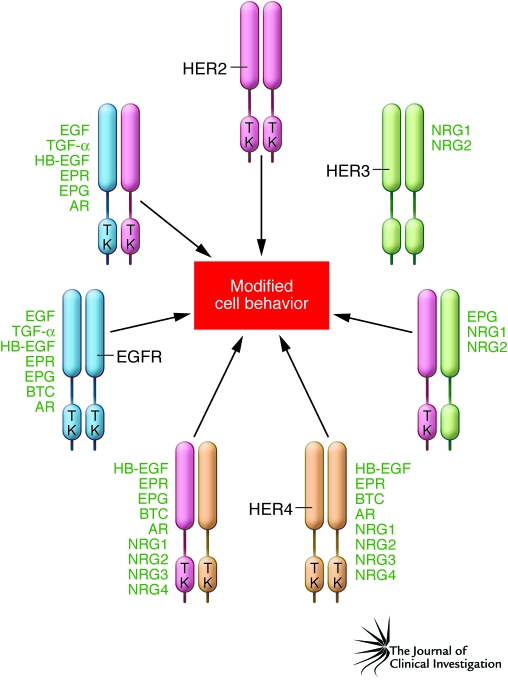
Figure 1. The HER family and its ligands.
There are four members of the HER family: EGFR (also known as HER1), HER2, HER3, and HER4. EGFR, HER2, and HER4 have a cytoplasmic tyrosine kinase (TK) domain that is activated by oligomerization. The HER3 kinase domain differs due to a lack of TK function. The minimal active configuration is either a homodimer or a heterodimer. HER family oligomerization is usually the result of binding by one of 11 types of ligand. This is even true for HER2, which is a coreceptor without a described soluble ligand. Oligomerization can also occur if either EGFR or HER2 is overexpressed or if activating mutations occur in their TK domain. Additional complexity is inherent in the HER system, since each receptor and many of the ligands can be products of alternative splicing, resulting in amplification of functional diversity. AR, amphiregulin; BTC, betacellulin; EPG, epigen; EPR, epiregulin; HB-EGF, heparin-binding EGF.