Figure 1.
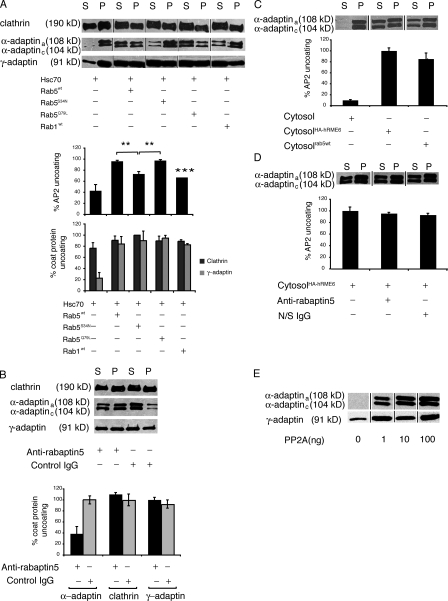
Rab5 specifically regulates AP2 uncoating in vitro. (A) Uncoating assays were performed on CCVs isolated from porcine brain incubated with ATP, Hsc70 (1.3 μg), and cytosols (50 μg) prepared from HEK293T cells overexpressing Rab5wt, Rab5Q79L, Rab5S34N, or Rab1wt as indicated. (top) CCVs (P) were separated from released coat proteins (S) and analyzed by Western blotting for clathrin using CHC 5.9, AP2 using anti–α-adaptin mAb 100/2, and AP1 using anti–γ-adaptin mAb 100/3. (bottom) α-Adaptin, clathrin, and γ-adaptin amounts released into supernatant during uncoating quantified by densitometry of Western blots (n = 3). Data expressed as a percentage ± SEM of maximal uncoating seen in the presence of rab5wt or rab5Q79L. AP2 uncoating in the presence of rab5S34N and rab1wt was significantly different from uncoating by rab5wt (**, P < 0.01) and rab5Q79L (***, P < 0.001). (B, top) HEK293T cytosol preincubated with anti-rabaptin5 antibodies to deplete rabex-5 or with nonspecific IgG was assayed for its ability to promote clathrin, AP2, and AP1 uncoating. (bottom) α-Adaptin present in supernatant was quantified and is expressed as a percentage of coat protein uncoating after treatment of cytosol with nonspecific IgG. Results are the mean ± range of duplicate samples. (C, top) Uncoating assays were performed in the presence of HEK293T cytosol or HEK293T cytosol overexpressing rab5wt or hRME-6. (bottom) α-Adaptin present in supernatants was quantified and expressed as a percentage of AP2 uncoating after incubation with cytosol overexpressing HA–hRME-6. Results are means ± range of duplicate samples. (D, top) Cytosol overexpressing hRME-6 was treated with anti-rabaptin5 antibodies or nonspecific IgG and assayed by Western blotting for its ability to uncoat AP2. (bottom) AP2 present in supernatants was quantified and expressed as a percentage of AP2 uncoating after incubation with control cytosol. Results are means ± range of duplicate samples. (E) Uncoating assays were performed in the presence of increasing concentrations of PP2A and supernatants were assayed for release of AP1 and AP2.