Figure 4.
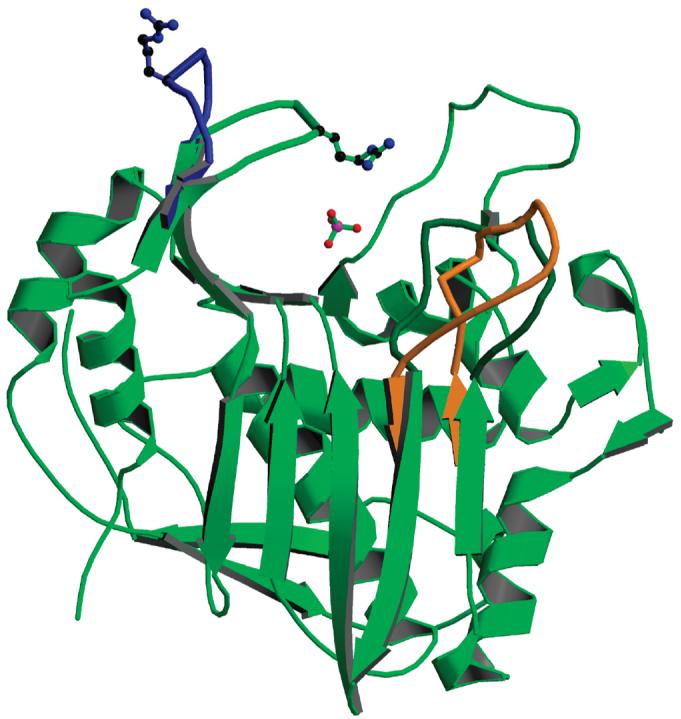
Subunit B, from the form 1 structure (in green), with superposed loop 45-56 (in blue) from subunit A of the same dimer. The side chain of Arg50 is shown for the two conformations. The phosphate ion is present in subunit B but not in subunit A; its position corresponds to the binding site of the phosphate of the dUMP substrate. Also shown is the position of loop 181-197 in the inactive conformation (in orange). Subunit A is the first crystallographic study of inhibitor-free hTS.
