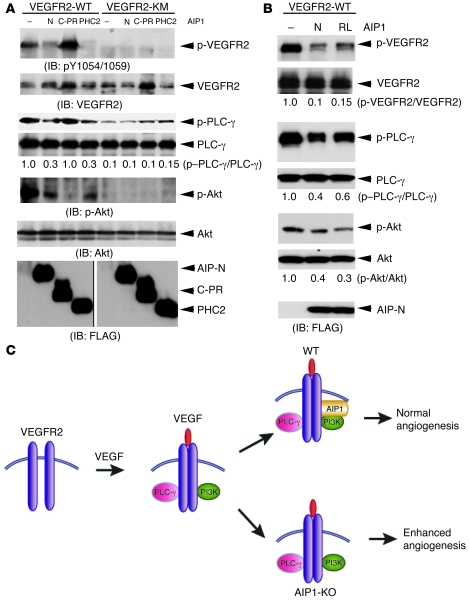
Figure 8. Mechanism for a negative regulation of VEGFR2 by AIP1.
(A and B) Effects of AIP1 mutants on VEGFR2-mediated signaling. VEGFR2-WT or a kinase-inactive mutant (KM) was cotransfected with various AIP1 mutants (AIP1-N, AIP1-C-PR, and PHC2 in A and AIP1-N and AIP1-N-R289L [RL] in B) into 293T cells. Phospho-VEGFR2 (pY1054/1059) and total VEGFR2, PLC-γ, and Akt were determined by respective antibodies. Relative ratios of p-VEGFR2/VEGFR2, p-PLC-γ/PLC-γ, and p-Akt/Akt are shown, with untreated WT as 1.0. Expression of AIP1 mutants was determined by Western blot with anti-FLAG. Note that the 2 FLAG blots were run on the same gel but noncontiguously as indicated by a black line. Association of AIP1 with various VEGFR2 was determined by immunoprecipitation with anti-VEGFR2, followed by Western blot with anti-FLAG. (C) A model for AIP1 as an endogenous regulator in VEGFR2-mediated signaling. VEGF rapidly induces VEGFR2 dimerization and autophosphorylation, followed by the recruitment and activation of PLC-γ and PI3K, leading to activation of PLC-γ–ERK, PI3K/Akt, and angiogenesis. AIP1 is recruited to the VEGFR2-PI3K complex in response to VEGF response. AIP1 via its C2 domain associates with VEGFR2 while via its PR domain binds to the SH3 domain of PI3K p85 to switch off VEGFR2 activation, leading to normal angiogenesis. In the absence of AIP1, VEGFR2-mediated angiogenic responses are enhanced.