FIG. 7.
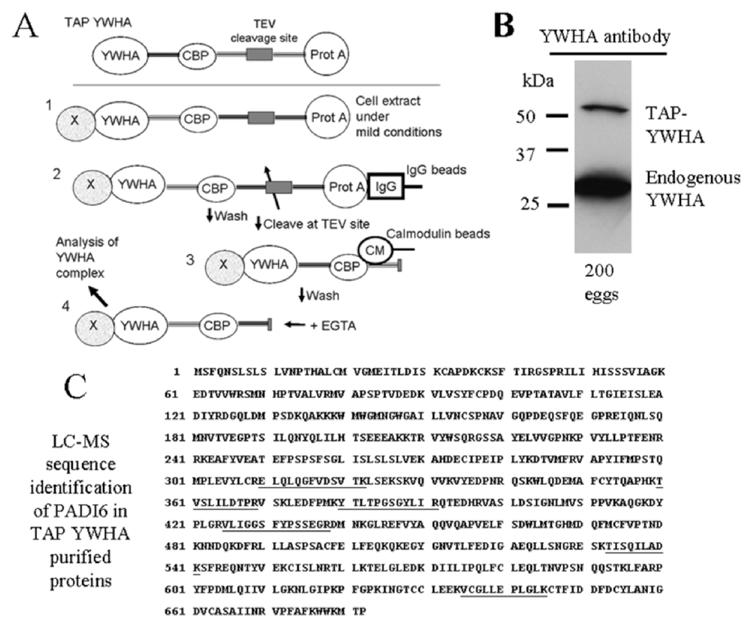
Procedures for the TAP of YWHA binding partners, TAP-YWHA expression, and LC-MS analysis. A) The TAP tag consists of dual tag of protein A from Staphylococcus aureus (Prot A) and calmodulin binding peptide (CBP): 1) In the first step of purification, cell extract containing TAP-YWHA and bound proteins is obtained under mild conditions. 2) The affinity for protein A to IgG is exploited to immobilize the TAP-YWHA to IgG-sepharose. Complexes are washed to remove proteins binding nonspecifically, and the CBP-YWHA is released by incubating the beads with TEV protease. 3) CBP is bound to calmodulin beads in presence of calcium. 4) Finally, the fusion protein, along with its binding partners, is eluted in a buffer containing EGTA, and the complex is characterized. B) Extracts of 200 eggs were prepared from transgenic mice known to contain the TAP-tagged YWHA by PCR. These extracts were resolved on a gel and probed in a Western blot with an anti-YWHA antibody to confirm that the eggs contained expressed TAP-YWHA. In these experiments, the anti-YWHA antibody labels any endogenous YWHA as well as the TAP-YWHA, which is detected at a higher molecular weight because it has the TAP construct associated with it. C) Results of the LC-MS indicate that the final eluate from the TAP-YWHA purification of 2400 mature eggs contained PADI6. The TAP-YWHA associated proteins were separated by SDS-PAGE, silver-stained, and sequenced by mass spectroscopic analysis. Sequencing data indicated that PADI6 was present in the band at approximately 75 kDa. Microsequenced peptides covered 10% of the mouse PADI6 sequence shown (UniProt primary accession no. Q8K3V4) with 100% identity (underlined portions).
