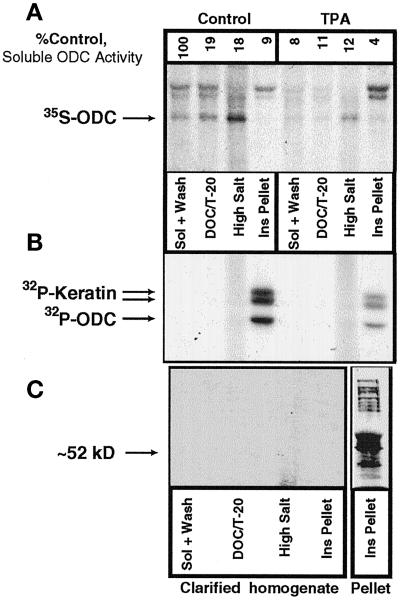
Figure 7.
Subcellular fractionation of radiolabeled ODC. (A) [35S]methionine-labeled NHEK lysate that had been treated with vehicle or 100 ng/ml TPA for 6 h was fractionated by detergent and salt extractions. Immunoprecipitable ODC protein was detectable in all fractions, with TPA treatment decreasing ODC levels, as we have reported previously (Ruhl et al., 1994). (B) NHEK were then labeled with [32P]orthophosphate and fractionated, and ODC was immunoprecipitated. Three bands were visible in the Ins Pellet fraction, one at the appropriate molecular size for ODC and the other two for keratins. (C) To determine whether the presence of the keratin bands was caused by specific interactions with ODC, the insoluble protein in the fractions was pelleted by ultracentrifugation, and ODC was immunoprecipitated from the resulting supernatant. No ODC was immunoprecipitated after this procedure. The lack of signal was not caused by degradation of the extract, as is shown in the Pellet column in which multiple phosphoproteins are apparent. Sol, soluble.