Abstract
The Ca2+ channel β-subunits, encoded by CACNB genes 1–4, are membrane-associated guanylate kinase (MAGUK) proteins. As auxiliary subunits of voltage-gated Ca2+ channels, the β-subunits facilitate membrane trafficking of the pore-forming α1 subunits and regulate voltage-dependent channel gating. In this report, we investigate whether two zebrafish β4 genes, β4.1 and β4.2, have diverged in structure and function over time. Comparative expression analyses indicated that β4.1 and β4.2 were expressed in separable domains within the developing brain and other tissues. Alternative splicing in both genes was subject to differential temporal and spatial regulation, with some organs expressing different subsets of β4.1 and β4.2 transcript variants. We used several genomic tools to identify and compare predicted cDNAs for eight teleost and five tetrapod β4 genes. Teleost species had either one or two β4 paralogs, whereas each tetrapod species contained only one. Teleost β4.1 and β4.2 genes had regions of sequence divergence, but compared with tetrapod β4s, they exhibited similar exon/intron structure, strong conservation of residues involved in α1 subunit binding, and similar 5′ alternative splicing. Phylogenetic results are consistent with the duplicate teleost β4 genes resulting from the teleost whole genome duplication. Following duplication, the β4.1 genes have evolved faster than β4.2 genes. We identified disproportionately large second and third introns in several β4 genes, which we propose may provide regulatory elements contributing to their differential tissue expression. In sum, both mRNA expression data and phylogenetic analysis support the evolutionary divergence of β4.1 and β4.2 subunit function.
Keywords: zebrafish, β-subunit, heart, nervous system, development, splicing, expression, membrane-associated guanylate kinase
the ca2+ channel β-subunits are auxiliary proteins of voltage-gated Ca2+ channels. Homology modeling and X-ray crystallography data indicate that the four mammalian Ca2+ channel β-genes (CACNB1–CACNB4, or β1–β4) belong to the MAGUK (membrane-associated guanylate kinase) family of scaffolding proteins. MAGUK proteins are composed of core src homology domain 3 (SH3) and guanylate kinase (GK) domains joined by a variable linker peptide (HOOK) and flanked by variable NH2- and COOH-terminal domains (22, 67). MAGUK proteins play important but diverse roles in protein trafficking, complex assembly, channel modulation, and gene expression (38, 40).
The β-subunits have two well-characterized cellular functions: they chaperone the pore-forming α1 subunit to the cell membrane for Ca2+ channel assembly, and they set voltage parameters for channel opening and closing (gating). The binding of the β-subunit GK domain to the highly conserved cytoplasmic linker (termed the α-interaction, or AID, domain) of the Ca2+ channel α1 subunit is critical for its modulation of channel currents (54). In addition, the intracellular binding of the β-subunits to the α1 AID domain masks an endoplasmic reticulum retention signal, which facilitates the release of the α1 subunit from the endoplasmic reticulum and its subsequent transport to the plasma membrane (15, 27, 63). β-Subunits can also operate independently of voltage-gated Ca2+ channels, potentially via the interaction of their highly conserved SH3 and GK domains with other, non-Ca2+ channel proteins in the cell (37, 56). The investigation of the broader range of β-subunit functions represents an exciting new area of research in the field of β-subunit biology.
As a family, the mammalian β-genes are clearly under complex temporal and spatial regulation during development. Each organ or tissue expresses a unique assemblage of β-genes (often restricted to particular splice variants), and this mix alters as development proceeds from embryonic to postnatal, juvenile, and adult stages (24, 35, 41, 68). The β4 subunit is typical of the β-genes in that it undergoes subtle changes in mRNA distribution as well as in absolute levels as development proceeds. During morphogenesis of the mouse central nervous system (CNS), β4 is initially expressed in the mantle zone throughout the entire brain and spinal cord at embryonic day (E) 13–E15 (62). Postnatally, β4 expression quickly attenuates in the brain with the exception of the cerebellum and olfactory bulb (62). In adults, β4 is again expressed broadly throughout the brain but retains prominent expression in the cerebellum (45). In the transition from newborn to adult, β4 subunit protein levels in the brain increase 10-fold, which represents the most dramatic increase of any β-subunit during this period (68). In the embryonic heart, β4 was first detectable in the myocardium at E9.5 (chamber morphogenesis stage) (7). Initial β4 expression was weaker in the atrioventricular canal and outflow tract relative to the developing chamber myocardium (7). However, in the adult heart, β4 gene expression is homogeneous in the working myocardium and conduction system (7). Additional adult tissues expressing β4 include the olfactory bulb, kidney, testes, retina, and lymphatic cells (31, 45). In chick, a truncated β4c variant expressed in brain and cochlea was found to localize to the nucleus and to interact with a nuclear protein involved in gene silencing and chromatin regulation (37). Understanding the physiological significance of the β-subunit diversity and how it relates to development remains a challenge.
Paradoxically, strong loss-of-function mutations reported for the β4 gene have no obvious embryonic phenotypes. In the lethargic mouse, the β4 coding sequence is altered by a 4-base pair (bp) insertion, which causes a reading frame shift and premature truncation of the β4 protein (20). This probable null allele leads to complex phenotypes in the adult mouse involving ataxia, lethargic behavior, and seizures (20, 47). Mutations in β4 are associated with epilepsy in humans (30). Although β4 is expressed in both embryonic and adult heart, no cardiac phenotypes have been reported thus far for the lethargic mutant.
We previously reported that two β4 genes, β4.1 and β4.2, exist in zebrafish. Both genes are expressed both maternally and zygotically in cleavage stage embryos (29). Loss-of-function studies using morpholinos revealed essential Ca2+ channel-independent roles for both β4 genes in gastrulation and early morphogenesis (29). Thus, the zebrafish appears to be an advantageous model system for the study of embryonic functions of the β4 proteins. An important next step in the analysis is to understand how (or whether) the expression and functions of the β4.1 and β4.2 genes differs as development proceeds. Over evolutionary time, each of the two genes might have adopted portions of the ancestral β4 function (subfunctionalization) or even adopted new functions (neofunctionalization) (49, 65).
In the present study, we investigated the hypothesis that β4.1 and β4.2 have adopted functionally distinct roles by undertaking a detailed comparison of their spatial and temporal expression patterns in embryo and adult. In addition, we isolated several predicted β4 gene sequences from four additional teleost species and five phylogenetically diverse tetrapod species. Using phylogenetic analysis, we assigned teleost genes to the β4.1 or β4.2 families. Comparative genomic analysis of the 13 vertebrate β4 genes allowed us to assess conserved functional domains and to compare the rates of molecular evolution of β4.1 vs. β4.2 genes. Overall, data from both expression and genomic analyses support the view that the β4.1 and β4.2 genes are indeed undergoing divergence in expression and function.
MATERIALS AND METHODS
Zebrafish strains and care.
This study used the WIK zebrafish strain. Zebrafish care was provided in accordance with animal care policies of Colorado State University. Embryos were staged as described (43).
Reverse transcription assays.
RNA was extracted using the total RNA extraction kit (Gentra) and stored at −80°C. To extract RNA from the 72 hours postfertilization (hpf) embryonic heart, we made use of the cardiac-specific cmlc2:GFP transgenic line (21). RT-PCR was performed for β4.1 and β4.2 genes using the Access RT-PCR System (Promega). The thermal cycling program was as follows: 48°C for 45 min (reverse transcription), followed by 94°C for 2 min (activation of PCR enzyme) and 40 cycles of 94°C for 30 s, 60°C for 1 min, and 68°C for 2 min. A final step of 68°C for 7 min allowed for a final extension. The primers used to distinguish the NH2-terminal and HOOK domain variants of β4.1 or β4.2 are given in Table 1. Electrophoresis of the final product was performed on a 2% agarose gel containing ethidium bromide. Gels were imaged using a digital camera and imaging software (Scion, Frederick, MD).
Table 1.
Primer sequences for Danio rerio CACNB4 genes
| Gene | Primer | Sequence |
|---|---|---|
| β4.1 | F69 | CCCCAGTCCCCTGAAGCTGGAGAAT |
| R70 | CAGGGATGGACCAACCAGAACCACA | |
| R90 | AAGGCTGGATCTGGTGTTTG | |
| F99 | TGTACCTGCATGGATTTGAAGACTCG | |
| F100 | GATCTGATGGCAGCACCACCTCCAC | |
| β4.2 | F71 | ATCGGCCGACTGGTGAAGGAAGGTT |
| R72 | TCCCTTTAGTGATGGGCCAACTAGCA | |
| R92 | CAAAGAGGGCTTTCTGCATC | |
| F101 | TGTACCTGCATGGATTTGAAGACTCG | |
| F102 | CGCAGCCGACTTAAGAGATTCTGATGG | |
| Control | EF1α F2 | CGGTGACAACATGCTGGAGG |
| EF1α R2 | ACCAGTCTCCACACGACCCA |
Whole mount in situ hybridization.
Digoxigenin-labeled anti-sense riboprobes (0.5 −1 kb) corresponding to the 3′ untranslated sequences of β4.1 and β4.2 mRNA were synthesized using T7 RNA polymerase from TOPO/TA based cDNAs as described (66). Embryos were fixed in 4% paraformaldehyde and perfused successively with probe, anti-digoxigenin and alkaline phosphatase-conjugated secondary antibodies. Signal was developed from NBT/BCIP substrate. Sense probes showed no signal. Whole mount specimens were photographed with a Spot Insight IN1120 digital camera on an Olympus SZX12 stereo-microscope.
Comparative genomics.
We used human and zebrafish β4 sequences to identify homologous sequences in the National Center for Biotechnology Information (NCBI), UCSC Genome, and Ensembl genome databases (1, 3–6). Since the UCSC Genome Browser provided the largest and most complete genomic contigs, data from these BLAT alignments were used to generate intron sizes shown in Fig. 4, and to determine conserved splice donor and splice acceptor sites (6). All stickleback (Gasterosteus aculeatus), medaka (Oryzias latipes) and pufferfish (Takifugu rubripes and Tetraodon nigroviridis) β4 exons were confirmed by contigs in NCBI and submitted as inferential sequences to the Third Party Annotation (TPA) database (Table 2). In Fig. 3B, the T. rubripes β4.1 exon 1b (which is not included in the TPA sequence since it is not found in GenBank) is derived from UCSC Genome Browser (chr Un:217332100-217332038); note all other β4.1 exons map to the same T. rubripes contig. Teleost expressed sequence tag (EST) sequences from medaka, and stickleback were obtained by searching the NCBI EST or WGS databases, JGI, or the UCSC Genome Browser (2) (1, 3, 5, 6). Although these EST sequences were not sufficient to define entire cDNAs, they were aligned with the other teleost sequences (not shown) and provided confirmation of the predicted teleost β4.1 and β4.2 cDNA sequences in regions where they differ from Danio rerio. Similar homology searches to genomic databases were used to identify a 3′ end for the β4 chicken (Gallus gallus) gene and to correct the 5′ exons (exons 1b, 2b and 2a) for the Anolis carolinensis, Monodelphis domestica, Ornithorhynchus anatinus, and G. gallus β4 genes.
Table 2.
GenBank Third Party Annotation accession numbers
| Species | Gene (alternate designation) | Accession No. |
|---|---|---|
| Takifugu rubripes | CACNB4.1_tv1 (β4.1a) | BK006576 |
| CACNB4.1_tv2 (β4.1b) | BK006577 | |
| CACNB4.2_tv1 (β4.2a) | BK006578 | |
| CACNB4.2_tv2 (β4.2b) | BK006579 | |
| Tetraodon nigroviridis | CACNB4.1_tv1 (β4.1a) | BK006580 |
| CACNB4.2_tv1 (β4.2a) | BK006581 | |
| CACNB4.2_tv2 (β4.2b) | BK006582 | |
| Gasterosteus aculeatus | CACNB4.1_tv1 (β4.1a) | BK006583 |
| CACNB4.1_tv2 (β4.1b) | BK006584 | |
| Oryzias latipes | CACNB4.1_tv1 (β4.1a) | BK006585 |
| CACNB4.1_tv2 (β4.1b) | BK006586 | |
| Anolis carolinensis | CACNB4_tv1 (β4a) | BK006587 |
| CACNB4_tv2 (β4b) | BK006588 | |
| Monodelphis domestica | CACNB4_tv1 (β4a) | BK006589 |
| CACNB4_tv2 (β4b) | BK006590 | |
| Ornithorhynchus anatinus | CACNB4_tv1 (β4a) | BK006591 |
| Gallus gallus | CACNB4_tv1 (β4a) | BK006592 |
| CACNB4_tv2 (β4b) | BK006593 |
Phylogenetic analysis.
Phylogenetic analyses were carried out to 1) assign β4 sequences to the correct paralog groups (β4.1 vs. β4.2) and 2) look for divergent patterns of molecular evolution between paralog groups that would be consistent with divergent gene function following gene/genome duplication. Amino acid sequences representing the core domain (“GSAD” in exon 3 through “WRAT” in exon 13) were aligned using ClustalW. Varying gap opening and extension parameters; regions for which reliable homology could not be established because of indels were excluded from analysis. Maximum likelihood analyses were implemented in TreePuzzle using the Müller & Vingron (VT) model of amino acid substitution with a mixed model of rate heterogeneity. Amino acid frequencies, the Γ distribution parameter α, and the proportion of invariant sites were estimated from the data. Nodal support was assessed using quartet puzzling. Equally weighted maximum parsimony analyses were carried out using PAUP*. A heuristic search was performed with 100 random addition replicates and TBR branch-swapping. Bootstrap proportions for clades were assessed with 1,000 pseudo-replicates. Bayesian analyses were carried out using MrBayes v3.1.2., specifying an invariant + Γ model of among-site rate heterogeneity and averaging over 10 models of amino acid substitution. Metropolis-coupled Markov chain Monte Carlo analyses were run for three million generations; the first million generations were discarded as burn-in. Resulting trees were rooted with the five tetrapods (A. carolinensis, O. anatinus, H. sapiens, M. domestica, and G. gallus) to allow comparison of evolutionary rates between the two fish paralog groups.
Accession numbers are for D. rerio EF601057, EF601060, EF601061 EF601062; for H. sapiens NP_001005747.1, NP_000717.2. See also Table 2.
RESULTS
Embryonic expression of the β4.1 and β4.2 genes is spatially and temporally restricted.
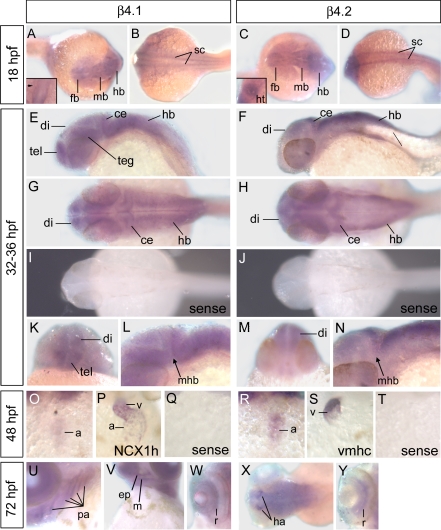
We used whole mount in situ hybridization to investigate the extent of overlap between β4.1 and β4.2 expression in the developing zebrafish embryo. We previously reported that mRNA for both β4 genes was present in the blastoderm and yolk syncytial layer of embryos at the 5-somite stage (∼12 hpf) and younger (28). Extending this analysis, we show that in segmentation stage embryos (10–22 hpf), β4.1 and β4.2 transcripts were primarily restricted to the developing CNS, including the two bilateral lines of spinal cord neurons extending from the hindbrain through the trunk and tail, as well as the forebrain, midbrain, and hindbrain ventricles (Fig. 1, A–D). Expression in the spinal cord neurons of the trunk and tail was strongly downregulated by the pharyngula stage (beginning ∼24 hpf) (Fig. 1, E and F). By 32–36 hpf, differential patterns of CNS expression were apparent between the two genes: β4.1 was expressed in the medial CNS of the hindbrain, while β4.2 was expressed more laterally in the hindbrain (Fig. 1, G and H). In addition, β4.1 expression predominated over β4.2 in the telencephalon and tegmentum, but β4.2 expression predominated in the diencephalon (Fig. 1, K–N).
Fig. 1.
Whole mount in situ hybridization indicating embryonic expression of β4.1 and β4.2. To minimize cross-reactivity between the two genes, antisense probes were designed to regions of 3′ untranslated sequence not significantly conserved between the β4.1 and β4.2 loci. A–D: at 18 hours postfertilization (hpf), expression occurs in the spinal cord (sc), forebrain (fb), midbrain (mb), hindbrain (hb), and yolk syncytial layer (ysl). Insets in A and C show dorsal views of the cardiac cone. In A, inset, β4.1 is not expressed strongly in the cardiac cone (arrowhead), prior to heart tube elongation, whereas (C, inset) strong β4.2 expression is detected in the heart (ht). E, F: central nervous system expression in the trunk decreases by 24 hpf. G, H, K, L, M, N: widespread but differential expression is detected in the brain at 32–36 hpf, specifically in the diencephalon (di) and telencephalon (tel), tegmentum (teg), cerebellum (ce), midbrain-hindbrain boundary (mhb), and the hindbrain. I, J: sense probes (negative controls) for β4.1 and β4.2, respectively, produced no signal. O, R: cardiac expression in 48 hpf embryos was primarily limited to the atrium (a). P, S: for comparison, sodium calcium exchanger, heart (NCX1h) expression occurs in both chambers, whereas ventricle myosin heavy chain (vmhc) expression is limited to the ventricle. U–Y: at 72 hpf, embryos express β4.1 in the pharyngeal arches (pa), including the mandibular arch (m) and ethmoid plate (ep), β4.2 in the habenular nuclei (ha), and both genes in the retina (r).
In addition to the CNS, β4 gene expression was detected in the embryonic heart (Fig. 1, O and R). While the primary adult cardiac β-subunit is β2 (24, 51, 71), the fetal hearts of mice and rats express both β2 and β4 genes (7, 35). Surprisingly, the earliest detection of β2 and β4 in mouse was in the linear heart tube undergoing chamber morphogenesis (E9.5)(7). In contrast, we detected cardiac expression of β4.2 much earlier, at the 21-somite stage (20 hpf), the time when bilateral cardiomyocyte precursors have met at the midline and begun to coalesce to form the cardiac cone, which eventually elongates to create the heart tube (Fig. 1C, inset). As hearts undergo looping morphogenesis at 48 hpf, the β4.1 and β4.2 genes showed strongest expression in the atrium, (Fig. 1, O and R).
At 72 hpf, additional tissue specific differences were apparent between β4.1 and β4.2 expression. β4.1 expression in the pharyngeal arches was more extensive than β4.2, although both are expressed in the mandibular arch (Fig. 1, U and V). In contrast, the β4.2 gene showed strong expression in the habenular nuclei of the epithalamus, visible as two prominent clusters of cells in the dorsal diencephalon (Fig. 1X). The habenular nuclei serve as a relay center that receives input from the telencephalon and projects to the interpeduncular nucleus located in the ventral midbrain (12). The habenular circuitry is well conserved and has been linked to complex behaviors including olfactory perception, feeding, mating, avoidance, and reward (60).
Finally, β4 gene expression was also detected in the retina (expressing both β4.1 and β4.2) (Fig. 1, W and Y). The expression of β4.2 and β4.1 within the retina at 72 hpf is consistent with the location of the ganglion cell layer and the inner nuclear layers (16, 31, 58). Functional studies have indicated the requirement for the β4, β2, and β3 subunits in the retina of postnatal or adult mice (11, 26, 46), though which β-subunits are functionally most important in the developing eye is unknown.
Expression of β4.1 and β4.2 in the embryo differs from that of another calcium channel subunit that has been described for zebrafish, the CACNB1 (β1) gene. Prior to the 19 somite stage, β1 expression within the CNS is restricted to the trigeminal ganglia, and expression in the skeletal muscle is prominent (66). Later in development, β1 expression also occurs in the telencephalon, diencephalon, tegmentum, and hindbrain in partially overlapping domains with β4.1 and β4.2, but β1 was not notably expressed in the cerebellum (74).
Overall, the zebrafish β4 genes were expressed in several of the same tissues as mammals, including midbrain, cerebellum, habenula, heart and retina (31, 45, 62). However, in zebrafish many of these tissues (including telencephalon, diencephalon, tegmentum, and habenula) expressed primarily only one β4 gene, consistent with possible subfunctionalization of β4.1 and β4.2. In addition, zebrafish expressed β4 genes in the mandibular and other pharyngeal arches, regions for which mammalian β4 expression have not been previously reported. Expression in these tissues may therefore represent neofunctionalization of the zebrafish paralogs, although this pattern is also consistent with loss of an expression domain in the mammalian β4 ortholog.
Developmental regulation of alternative splicing in β4.1 and β4.2.
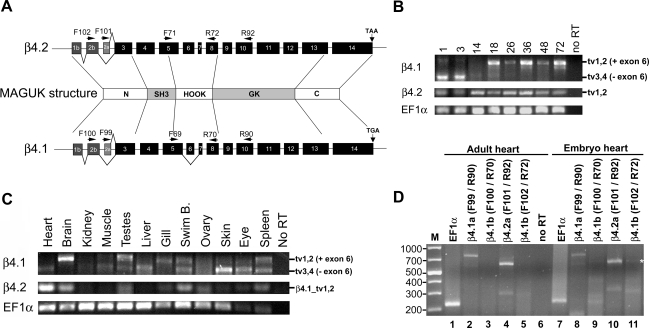
The zebrafish β4.1 gene (but not β4.2) undergoes alternative splicing within the HOOK domain, in addition to the NH2 terminus (Fig. 2A) (28). To investigate the developmental expression of full-length or shortened β4.1 and β4.2 variants, we performed RT-PCR using primers that flank exon 6 in the HOOK domain (Fig. 2A). For β4.1, primers in exons 5 and 10 amplify different sized products from full-length transcript variants that retain exon 6 (β4.1_tv1 and β4.1_tv2) and transcript variants lacking exon 6 (β4.1_tv3 and β4.1_tv4). For β4.2, primers in exons 5 and 8 would yield only a single product since this gene is not subject to alternative splicing in the HOOK domain.
Fig. 2.
Transcript variant-specific regulation in development and in adult tissues. A: diagram of β4.1 and β4.2 gene structure, showing primer locations and alternative splicing. MAGUK, membrane-associated guanylate kinase. B–D: RT-PCR of RNA transcripts using primers located flanking the internal spliced exon 6 (primers F71 and R72 for β4.2; and primers F69 and R90 for β4.1). β4.1_tv1 and β4.1_tv2 variants retain exon 6 (384 bp amplicon); β4.1_tv3 and β4.1_tv4 variants lack exon 6 (335 bp amplicon). β4.2_tv1 and β4.2_tv2 variants are not alternatively spliced in the HOOK domain (279 bp) B: developmental time course. RNA from embryos at several developmental time points was assayed (presented in hpf). Lanes 1 and 3 (hpf) represent an independent gel from lanes 14–72 (hpf). C: adult tissues. D: expression in the embryonic vs. adult heart, using primers specific for each splice variant. Lanes 1 and 7: EF1α positive controls (220 bp). Lanes 2 and 8 (β4.1a transcripts): β4.1_tv1 (734 bp), and β4.1_tv3 (684 bp) products both present. Lanes 3 and 9 (β4.1b transcripts): Neither β4.1_tv2 (566 bp) and β4.1_tv4 (516 bp) products were detectable. Lanes 4 and 10 (β4.2a transcript): β4.2_tv1 (609 bp) product was present. Lanes 5 and 11 (β4.2b transcript): β4.2_tv2 (600 bp) product was present in embryo (*) but not adult RNA. Lane 6: no reverse transcriptase (RT) negative control.
In RNA extracted from 1 or 3 hpf embryos, the shorter transcript variant predominated, although some full-length transcript variants also amplified (Fig. 2B). Since zygotic expression initiates in zebrafish around the 512-cell stage (2.75 hpf), amplification from 1 hpf extracts must be due to the presence of maternally provided β4 RNA. Maternal expression of a Ca2+ channel β-subunit was previously reported in ascidians (TuCavbeta) (50). In addition, the zebrafish Ca2+ channel β2 (CACNB2) genes, β2.1 and β2.2, are also expressed maternally (28). The β4.1 short transcripts (lacking exon 6) continued to be expressed throughout epiboly and into the tail-bud stages (10 hpf), but were not detectable later (Fig. 2A and data not shown). Full-length β4.1 transcripts were barely detectable in gastrulation and early segmentation stages but became more abundant in segmentation and pharyngeal stages (18–72 hpf) (Fig. 2B). In comparison, β4.2 transcripts were expressed steadily from 1 hpf through 72 hpf. Therefore, as in mammals, the developmental regulation of splice variants increases the diversity of expressed β4 transcripts.
Regulation of β4.1 and β4.2 alternative splicing in the adult.
We also observed differential expression of β4.1 transcript variants in the adult. Several organs or tissues, including the heart, brain, liver, gill, skin, and eye, expressed one β4.1 variant (full-length or internally shortened) more strongly than the other (Fig. 2C). Notably, the adult heart and brain showed a striking reciprocal expression pattern of β4.1 transcripts, with heart expressing predominantly the full-length transcript and brain expressing predominantly the shorter form. However, the muscle, testes, swim bladder, and spleen expressed both β4.1 transcripts. The β4.2 gene was detected strongly in heart and brain, with less expression in testes, swim bladder, ovary, and spleen. The kidney was the only adult organ for which we failed to detect either β4.1 or β4.2 expression. In mammalian adults, β4 gene expression has been reported for heart, brain, testes, eye, kidney, and lymphocytes (10, 11, 31, 37, 72). Thus, zebrafish adults share several major sites of β4 expression with mammals (heart, brain, and eye), but fish also express β4 genes in some additional tissues so far not reported for mammals.
Comparative expression of β4 transcript variants in the embryonic vs. adult heart.
Since the heart is a major site of β4 expression in all vertebrates examined, we selected this organ for a detailed analysis assessing the presence of all known splice variants in embryo vs. adult. In vertebrates, alternative splicing among the 5′-most exons of the β4 genes gives rise to a shorter β4a transcript and a longer β4b transcript (36), reflecting alternative splicing within three exons (named 1b, 2b, and 2a as in Ref. 28; Fig. 5A). Amplifications used one NH2-terminal specific primer (located either in exon 2b or exon 2a) and one internal primer (located in exon 10 so as to be 3′ of alternatively spliced exon 6). Both β4.1a and β4.2a variants were expressed in embryos and adults. Of the β4b transcripts, only β4.2b was weakly detected in embryos. Somewhat surprisingly, the profile of expressed β4 transcript variants for the heart was exactly the same in embryo and adult, with the exception of β4.2b expression in the embryonic heart (Fig. 2D).
Identification and comparative analysis of vertebrate β4 genes.
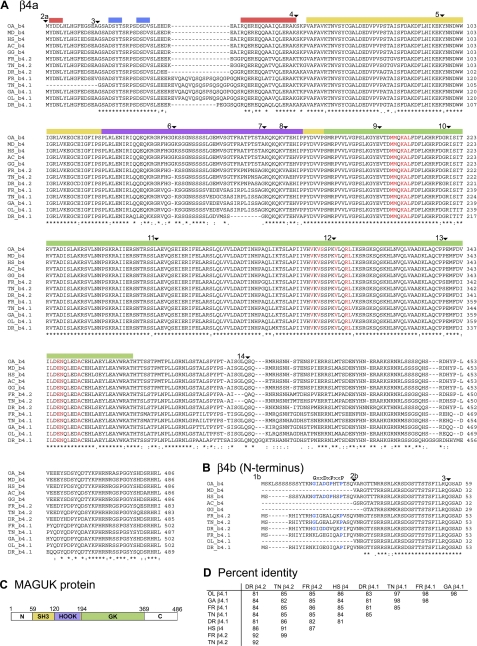
The second major objective of this study was to use comparative genomics to evaluate the levels of conservation of teleost β4.1 and β4.2 and tetrapod β4 through evolution. Accordingly, we performed TBLASTX, BLAT, and MEGABLAST searches of public sequence databases to identify the full sequences for four β4 genes in pufferfish–two each in the T. nigrovidis and T. rubripes genomes (1, 5, 6). The duplicated Tetraodon and Takifugu genes were designated as β4.1 or β4.2 based on the similarity of their predicted cDNA sequence to the zebrafish β4.1 and β4.2 genes (Fig. 3, A and B), as well as the relationships demonstrated by our phylogenetic analysis. In addition, we identified a single β4 gene each for medaka (O. latipes) and the three-spined stickleback (Gasterosteus aculeatus) in contiguous genomic sequences; both of these are β4.1 homologs based on our phylogenetic analysis. (While a second β4 gene may exist in these species, single contigs linking all exons are not yet available.) As expected, all exons of the six new teleost β4 genes were flanked by the consensus invariant residues expected for splice acceptor or donor sites (AG or GT) (data not shown). Exons 1b, 2b, and 2a are often missing or incorrectly identified by electronic annotation in current databases, probably because they are so short (only 45–84 bp) and frequently distantly located from exon 3 (see discussion on intron size below). However, we did ultimately identify homologs of exons 1b, 2b, and 2a on the same contig as the core exons for all of the teleost species except T. nigroviridis β4.1 and O. latipes β4.1, suggesting that this pattern of NH2-terminal alternative splicing is broadly conserved across vertebrates. Using similar methods, we corrected the electronic annotation of tetrapod β4 genes, although exon 1b could not be identified unambiguously for all genes.
Fig. 3.
Alignment of teleost and tetrapod β4 genes. A: ClustalW alignments of 13 vertebrate β4 genes of diverse lineages. The tetrapod sequences include 3 classes: Lepidosauria (Anolis carolinensis, AC, lizard), Aves (Gallus gallus, GG, chicken), and Mammalia. Within Mammalia, 3 orders are represented: Primates (Homo sapiens, HS, human); Metatheria (Monodelphis domestica, MD, opossum); and Monotremata (Ornithorhynchus anatinus, OA, platypus). The teleost species all belong to the class Actinopterygii. Species include: Oryzias latipes, OL, medaka; Gasterosteus aculeatus, GA, three-spined stickleback; Takifugu rubripes, FR, pufferfish; Tetraodon nigroviridis, TN, pufferfish; Danio rerio, DR, zebrafish. ▾ demarcates exon borders. Conserved regions of α-helix (red bars) or β-strand (blue bars) within the NH2 terminus are marked. The yellow, lavender, and green regions depict the SH3, HOOK, and GK domains, respectively. Amino acid residues shown in red are those found to interact with the α1 (CACNA) subunit in humans (22). B: ClustalW alignment of the alternatively spliced β4b NH2 terminus. For β4b, a proline-rich motif, GxxDxPxxP, potentially important in Cav2.1 gating, is indicated (36). These residues, if conserved in teleost species, are highlighted in blue. C: diagram of the MAGUK protein structure. N, NH2 terminus; C, COOH terminus.
All described teleost β4 genes encode predicted proteins similar in structure to zebrafish and human β4 subunits, including the highly conserved SH3 and GK domains characteristic of MAGUK family proteins (Fig. 3C). The size and number of exons comprising these domains are nearly identical in teleosts and mammals (Fig. 3, A and B). In addition, the phase of the exons in splicing (relative to the open reading frame) was nearly identical for all 13 β4 genes (data not shown). The MAGUK “core” (SH3-HOOK-GK domains) was highly conserved, ranging from 81 to 87% amino acid identity compared with human, but higher (91–99%) when compared with genes within the β4.1 or β4.2 paralog groups (Fig. 3D). Residues identified by crystallographic studies as important for interaction of the β-subunit with the α-subunit were 100% conserved in the 13 β4 genes (24). The COOH terminus (exons 13 and 14) was the most divergent region of the teleost and tetrapod β4 genes (Fig. 3A).
Recent resolution of the tertiary structure of the human β4a protein identified two regions of α-helix and two regions of β-sheet (Fig. 3A) (69). These regions are nearly 100% identical between humans and the other 12 sequences examined, suggesting strong conservation among vertebrates in both protein folding structure and function of the β4a NH2-terminal domain.
The β4.1 and β4.2 sequences showed certain characteristic differences. Several of the teleost β4.1 genes (but not zebrafish) include a glutamine-rich sequence (EVQAQVQSQSPSQSQAPGQS) in exon 3 prior to the SH3 domain (Fig. 3, A and C). This sequence was not observed in the β4.2 genes or any of the tetrapod β4 genes, and its function is currently unknown. Moreover, the β4.1 genes and β4.2 genes were distinct within the COOH terminus, which is also the most divergent part of the β4 gene in tetrapods.
Human β4b transcript variants exhibit a proline-rich motif, GxxDxPxxP, spanning amino acids 10-20 of the NH2 terminus (36). Electrophysiological studies indicate this domain is critical for the distinct properties of β4b proteins in modulating Ca2+ channel slow inactivation and pharmacological block by the peptide ω-conotoxin (ωCTx)-MVIIC (36). Interestingly, the initial glycine (G10) and proline (P15) residues were not well conserved in the teleost β4.1 or β4.2 genes (Fig. 3B). However, in all 13 genes the final proline (P18) was 100% conserved and the aspartic acid (D13) residue was either conserved or replaced with glutamic acid (E13) (a conservative substitution).
Thus, despite the 418 million years since the last common ancestor of humans and teleost fish (8, 13), the genomic organization, secondary structure, splicing, and sequence of the β4 genes remain highly conserved, although β4.1 and β4.2 genes do show some distinctive sequences.
Comparative genomics.
Teleost fishes underwent a whole genome duplication event ∼300 million years ago, and this event has had a lasting effect on teleost developmental genetics (42, 52, 70). Specifically, this duplication event produced two identical, and functionally redundant, copies of all coding sequences in the genome. Such duplicate genes were either eliminated from the genome by mutational processes or maintained in the genome, taking on a new function (neofunctionalization) or a portion of the gene's original function (subfunctionalization) (49). If the two β4 genes we identified in zebrafish trace their origins to this whole genome duplication, we would expect to see two β4 paralogs in all teleost genomes, or a phylogenetic tree consistent with loss of one copy from some teleost lineages following whole genome duplication. Thus, we performed a phylogenetic analysis on teleost and tetrapod sequences to: 1) test whether the origin of the zebrafish β4 paralogs coincided with the teleost whole genome duplication event; 2) assign novel teleost sequences to the correct paralog group (β4.1 vs. β4.2); and 3) look for divergent patterns of molecular evolution between paralog groups consistent with divergent gene function (subfunctionalization or neofunctionalization) following gene/genome duplication.
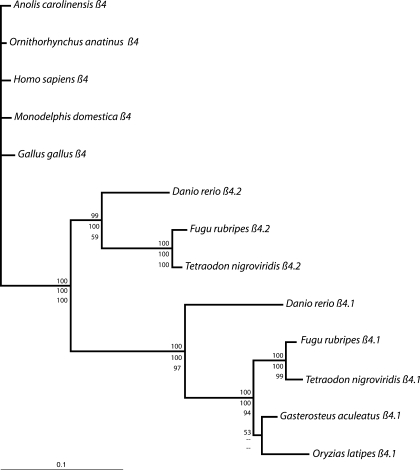
The total alignment of β4s was 513 amino acids in length; 32 positions that could not be unambiguously aligned were excluded from phylogenetic analysis. Maximum likelihood, maximum parsimony, and Bayesian analyses yielded well-supported congruent topologies. The resulting gene trees show three well-supported clades: 1) tetrapod β4s, 2) teleost β4.1s, and 3) teleost β4.2s, indicating that the two β4 paralog groups likely trace their origins to the teleost-specific whole genome duplication (Fig. 4). The single β4 genes identified from G. aculeatus and O. latipes are both β4.1s. Within each paralog group, relationships among the teleost lineages reflect currently accepted phylogenetic relationships.
Fig. 4.
Phylogenetic relationships among the β4 genes of 13 vertebrate species. Tree is rooted with the 5 tetrapods: O. anatinus, H. sapiens, M. domestica, A. carolinensis, and G. gallus. Topology and branch lengths result from a Bayesian analysis of amino acid data. Top numbers are posterior probabilities. Maximum likelihood and maximum parsimony analyses yielded congruent topologies; middle numbers are maximum likelihood quartet puzzling support values, and bottom numbers and maximum parsimony nonparametric bootstrap support values. Maximum likelihood analyses resolved the [G. aculeatus + (F. rubripes + T. nigroviridis)] node with a support value of 99. Branch lengths are much longer within the teleost clade than within the tetrapod clade, despite older divergence dates within tetrapods (13). Within teleosts, branch lengths are much longer within the β4.1 gene family than within the β4.2 gene family, indicating heterogeneous rates of molecular evolution among clades and between paralog groups.
Branch lengths are uneven throughout the tree, indicating varying rates of amino acid evolution between paralogs and among species. Specifically, amino acid substitution rates are higher among the teleost lineages than among the tetrapod lineages; substitution rates among tetrapods are sufficiently low to preclude phylogenetic resolution. Furthermore, within the teleosts, amino acid substitution rates are higher among the β4.2s than among the β4.1s, indicating different rates of molecular evolution in these two paralogs following the teleost-specific whole genome duplication.
Human chromosome 2 and mouse chromosome 2 share a region of synteny that links the β4 gene with the HOXD cluster (23, 31, 64). Upon noting that β1 is linked to the HOXB cluster, and β3 linked to the HOXC cluster, Escayg et al. (31) proposed that the β4 gene is part of an ancient linkage group with HOX genes. During the transition of the prechordate invertebrate to early vertebrates, the single cluster of HOX genes underwent two rounds of duplication, leading to the current four HOX clusters in mammals. The parallels in genomic structure of the four mammalian β-genes, and their similar sequence divergence, indicates their origin from a single ancestral β4 gene (31). In ray-finned fishes, an additional genome duplication event in the lineages leading to teleosts led to the further duplication of HOX clusters, resulting in the current seven zebrafish clusters (Aa, Ab, Ba, Bb, Ca, Cb, and D) (33, 53, 55). The duplicated HOXD cluster appears to have been lost during teleost evolution. We find that the zebrafish β4.2 gene has retained the ancient linkage to the HOXD cluster on chromosome 9, which contains a region syntenic with human chromosome 2 and mouse chromosome 2 (Table 3). However, the zebrafish β4.1 gene on chromosome 6 was not linked to any Hox genes or paralogs of other genes listed in Table 3.
Table 3.
Conserved linkage of CACNB4 genes with the HOXD gene family
| Duplicated Gene |
Species |
|||||
|---|---|---|---|---|---|---|
|
D. Rerio |
Homo Sapiens
|
Mus Musculus
|
||||
|
Chr 9 (0.67–43M) |
Chr 2 (118–192M) | Chr 2 (52–84M) | ||||
| Nuclear factor (erythroid-derived)-like 2 | Nfe2l2 | (0.67) | NFE2L2 | (177.80) | Nfe2l2 | (75.51) |
| Homeobox D4 | HoxD4 | (1.56) | HOXD4 | (176.72) | HoxD4 | (74.56) |
| Even-skipped homeobox 2 | Evx2 | (1.61) | EVX2 | (176.65) | Evx2 | (74.49) |
| Distal-less homeobox 2a | Dlx2a | (3.54) | DLX2 | (172.67) | Dlx2 | (71.38) |
| Integrin alpha v | Itgav | (10.20) | ITGAV | (187.16) | Itgav | (83.56) |
| GLI Krup̈pel family member GLI2 | Gli2a | (35.13) | GLI2 | (121.40) | ||
| Calcium channel β4 subunit | CACNβ4.2 | (36.01) | CACNβ4 | (152.40) | CACNβ4 | (52.27) |
| DEAD box polypeptide 18 | Ddx18 | (36.24) | DDX18 | (118.29) | ||
| Signal transducer and activator of transcription 4 | Stat4 | (39.61) | STAT4 | (191.60) | ||
| Neurogenic differentation 1 | NeuroD | (42.63) | NEUROD1 | (182.25) | NeuroD1 | (79.29) |
Large size of 5′ introns.
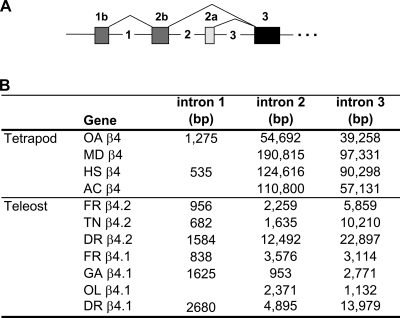
An unusual feature of the teleost β4 genes is the remarkably large size of some of the initial introns. Intron 2 is >3,000 bp in three teleost β4 genes. Intron 3 is >3,000 bp in five out of seven teleost genes, and >10,000 bp in three teleost species. For zebrafish and the two pufferfish species, the size of β4.1 and β4.2 introns 2 and 3 ranks them among the largest known introns in the genome (Fig. 5B) (8). In pufferfish, smaller intron size in general is correlated with the compact nature of the pufferfish genome, and 75% of introns are <425 bp in length (8). Thus, although the 5′ β4 introns of pufferfish are small compared with 5′ human β4 introns (Fig. 5B), they are very large for pufferfish. The human β4 intron 2 is 124,616 bp, and intron 3 is 90, 298 bp (36). In humans, the mean size of introns adjoining coding sequences is 3,749 bp, but 75% of introns are smaller than 2,609 bp (8, 39, 57). Large second and third introns were also observed in all other tetrapods examined (Fig. 5B). The sizes of other introns within the gene, including intron 1, were not larger than typically expected for the species. It was previously noted in several species that the median size of introns separating 5′ untranslated region (UTR, noncoding) sequence (∼8,223 bp), was significantly larger than a typical intron separating coding sequence (median size ∼3,000 bp) (39). However, this observation does not account for the large intron size in the β4 genes, since the large introns separate coding sequences in this case.
Fig. 5.
Genomic structure of teleost β4 genes. A: β4 gene structure, showing alternative splicing at the NH2 terminus to create β4b transcript variants or β4a transcript variants. In β4b transcripts, exons 1b and 2b splice to exons 3–14, whereas in β4a transcripts, exon 2a splices directly to exons 3–14 (Fig. 2A). The first 3 introns (not drawn to scale) are numbered. Arrows designate locations of RT-PCR primers used in expression studies. B: the percent amino acid identity in exons 3–14 for vertebrate species. C: the size of introns 1, 2, and 3 in β4 genes of several species.
DISCUSSION
This study utilized two independent approaches to investigate the potential for differential function of the β4.1 and β4.2 genes. We find that several of the zebrafish β4.1 and β4.2 transcript variants are differentially expressed during embryonic development and in multiple adult tissues. In addition, we report a phylogenetic analysis of 13 vertebrate β4 genes, including several β4 genes described completely for the first time in this report. This comparative genomic approach allowed us to assign the teleost β4.1 and β4.2 genes to the correct paralog group. We find that many aspects of β4 gene structure are highly conserved among the teleost and tetrapod β4 genes, but that the β4.1 and β4.2 gene families do have some sequence differences compared with each other. In addition, β4.1 genes are evolving more quickly than β4.2 genes. Thus, results from expression analyses and genomic analyses both support the position that the β4.1 and β4.2 genes are likely to have some functional differences.
The expression pattern of the β-genes in mammalian tissues is complex and changes considerably throughout development. In common with mammalian β4 genes, zebrafish β4.1 and β4.2 genes were expressed in the developing brain, eye, and heart. However, the predominant areas of expression within the developing brain were different for β4.1 and β4.2. These data are consistent with possible subfunctionalization between the two genes. In addition, some regions of β4.1 and β4.2 expression, such as the pharyngeal arches, were unique to teleosts. The function of the β4's in these tissues may represent neofunctionalization relative to mammals, although data from additional taxa is required to eliminate the possibility that pharyngeal arch expression was lost in mammals rather than gained in zebrafish.
We selected the heart for a more detailed analysis examining expression of all known zebrafish β4.1 and β4.2 splice variants. In keeping with the general theme of heterogeneous expression of β4 genes throughout development, we found that the embryonic and adult heart expressed only a subset of β4.1 and β4.2 variants. The β4a transcript (comprising the shorter NH2 terminus using exon 2a) were observed for β4.1 and β4.2, but no β4b variants (comprising the longer NH2 terminus using exon 1b and 2b) were observed. The exception was a barely detectable β4.2b expression in the embryonic heart only. Other than this single difference, the profile of β4.1 expression in embryonic hearts of 72 hpf was precisely the same as in adult hearts.
Making physiological sense of this diversity remains a challenge. The two best-demonstrated roles for the β-subunit are the facilitation of membrane expression of the pore-forming α1 subunit and the modulation of gating properties of assembled channels (α1, α2-δ, and β-subunits) (38). The β-subunit further increases the complexity of endogenous voltage-gated Ca2+ channel (VGCC) complexes by impacting the overall conformation of the complex (18) and affecting its distribution in the plasma membrane (19). With these roles in mind, a hypothesis gaining support is that the milieu of heterogeneous β-subunits may, through their unique functional attributes, differentially modulate or fine-tune VGCC currents during development (7, 24, 61). Supporting the idea that alternative splice variants have functional significance, Xenopus expression studies verified that VGCC currents are different when β4a vs. β4b variants were expressed (36). Thus, it was intriguing to observe that β4a variants specifically predominated in the zebrafish heart.
A second hypothesis regarding the potential significance of splice variants is that they have separable cell biological functions consistent with differential subcellular localizations. In support of this idea, several reports confirm that vertebrate β-transcript variants derived from the same gene localize to different regions within the cell (17, 19, 25, 37). As a remarkable example of novel localization, alternative splicing within the chick β4c variant within the HOOK domain causes the introduction of an early termination codon, leading to premature truncation of the protein. Thus, β4c encodes the NH2-terminal half of the protein but lacks the GK domain and its associated AID domain (37). Surprisingly, β4c localizes to the cell nucleus, where it interacts directly with a chromobox protein to attenuate gene silencing (37). Based on this and other reports, it is becoming apparent that the β-subunits perform tasks within the cell other than the well-defined roles in regulating voltage-gated Ca2+ channels (56).
The modular nature of the β4 proteins as MAGUK family members may contribute to their potential for diverse functions. The highly conserved SH3 and GK domains could facilitate interaction with proteins not associated with Ca2+ channels. Indeed, a recent report indicates that the β2 subunit SH3 domain participates in regulation of endocytic turnover of Ca2+ channels (and other membrane proteins) in a process dependent on the microtubule binding protein dynamin (34). The newly proposed multifunctional aspects of β-subunit biology suggest that much remains to be learned from the study of these proteins in the context of biological systems and that the genetic phenotypes should be investigated with the modular nature of the β-subunit proteins in mind.
The teleost fish account for about one-half of all extant vertebrate species, and they are widely divergent in morphology, behavior, ecology, and genomic organization (70). The whole genome duplication that occurred in teleosts ∼300 million years ago provided opportunity for diversification of gene function without the associated penalties of gene loss (32) (42). Gene duplication, followed by subfunctionalization or selective gene loss, has been proposed to facilitate extensive speciation (52, 59, 70). To assess the evolution of vertebrate β4 genes, we isolated sequences for several teleost genes for comparison to tetrapod genes of various phyla. Comparative analysis indicates that the patterns of alternative splicing are similar to human β4 and that exons are conserved in size and sequence. Key amino acid residues that interact with the α1 subunit are highly conserved. Our phylogenetic results indicate that the duplicate β4 genes in zebrafish likely resulted from the teleost-specific whole genome duplication. The β4.1 genes are evolving slightly faster than β4.2 genes, consistent with sub- or neofunctionalization.
An interesting trend of teleost and tetrapod β4 genes is that introns 2 and 3, which separate the short, alternatively spliced 5′ coding exons, are among the largest introns of the genome. Curiously, we also observed a similar trend for introns 2 and 3 in the β2 (CACNB2) genes of zebrafish, pufferfish, and human (28). The functional significance of these large introns, if any, is not known. However, we note that the introns 2 and 3 separate the exons used for mutually exclusive forms of the NH2 terminus (β4a vs. β4b transcript variants). As organisms become better studied at the genomic level, it is becoming evident that introns can impact mRNA metabolism in a number of ways. Potential effects include modulating transcription rates by regulating DNA accessibility, modulating editing, and polyadenylation of the pre-mRNA and affecting nuclear export, translation, and mRNA decay rates (44, 48). Conceivably, the large introns might incorporate enhancer or repressor elements that influence transcription of the β4 splice variants (44). Consistent with this hypothesis, the transcript variants of the zebrafish β4 and β2 genes are in fact subject to spatial and temporal regulation in both embryo and adult (Figs. 4 and 5 and Ref. 28). We speculate that these large introns provide a mechanism for independent cis-regulation of each transcript variant. Sequences within individual intron or 5′ UTR sequence may independently direct the tissue-specific or temporally specific expression of each transcript variant.
Mutations in several different Ca2+ channel α1 subunit genes have been shown to associate with inherited human disease (9, 14). Since the β-subunits play a critical role in the localization and function of mature VGCCs, they may also be considered attractive candidate genes for congenital disease. Mutation of β4 in humans is associated with epilepsy (20). Adult lethargic mice have neuropathological phenotypes involving seizures, but no embryonic phenotypes have been reported for this β4 mutant. In contrast, knockdown of either β4.1 or β4.2 is embryonically lethal in zebrafish (29). As reported here, the expression data and phylogenetic analyses discussed in this report are consistent with potential functional differences between the β4.1 and β4.2 genes. The zebrafish system offers the opportunity for detailed functional studies which may define the specific contributions of the β-subunits to the developing embryo.
GRANTS
Supported by National Institute of Neurological Disorders and Stroke Grant NS-42600 to W. A. Horne and National Science Foundation grants IOS-0719242 and IOS-0719083 to W. A. Horne and D. M. Garrity, respectively.
Acknowledgments
We thank Heather Hergert, Kiara Foltyn, and Kathryn Gately for outstanding technical support.
Address for reprint requests and other correspondence: D. M. Garrity, Dept. of Biology, Colorado State Univ., Ft. Collins, CO 80523 (e-mail: Deborah.garrity@colostate.edu).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.The Fugu Genome Project Database [http://www.fugu-sg.org/].
- 2.The JGI Eukaryotic Genomes Databases [http://genome.jgi-psf.org/].
- 3.The National Center for Biotechnology Information Entrez Database [http://www.ncbi.nlm.nih.gov/sites/gquery].
- 4.The Sanger Center Ensembl Databases [http://www.ensembl.org/index.html].
- 5.The Tetraodon Genome Browser [http://www.genoscope.cns.fr/externe/tetranew/].
- 6.The UCSC Genome Bioinformatics Databases [http://genome.ucsc.edu/].
- 7.Acosta L, Haase H, Morano I, Moorman AF, Franco D. Regional expression of L-type calcium channel subunits during cardiac development. Dev Dyn 230: 131–136, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Aparicio S, Chapman J, Stupka E, Putnam N, Chia JM, Dehal P, Christoffels A, Rash S, Hoon S, Smit A, Gelpke MD, Roach J, Oh T, Ho IY, Wong M, Detter C, Verhoef F, Predki P, Tay A, Lucas S, Richardson P, Smith SF, Clark MS, Edwards YJ, Doggett N, Zharkikh A, Tavtigian SV, Pruss D, Barnstead M, Evans C, Baden H, Powell J, Glusman G, Rowen L, Hood L, Tan YH, Elgar G, Hawkins T, Venkatesh B, Rokhsar D, Brenner S. Whole-genome shotgun assembly and analysis of the genome of Fugu rubripes. Science 297: 1301–1310, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Avanzini G, Franceschetti S, Mantegazza M. Epileptogenic channelopathies: experimental models of human pathologies. Epilepsia 48: 51–64, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Badou A, Basavappa S, Desai R, Peng YQ, Matza D, Mehal WZ, Kaczmarek LK, Boulpaep EL, Flavell RA. Requirement of voltage-gated calcium channel beta4 subunit for T lymphocyte functions. Science 307: 117–121, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Ball SL, Powers PA, Shin HS, Morgans CW, Peachey NS, Gregg RG. Role of the beta(2) subunit of voltage-dependent calcium channels in the retinal outer plexiform layer. Invest Ophthalmol Vis Sci 43: 1595–1603, 2002. [PubMed] [Google Scholar]
- 12.Bally-Cuif L Breaking symmetry on time. Dev Cell 12: 1–2, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Benton MJ, Donoghue PC. Paleontological evidence to date the tree of life. Mol Biol Evol 24: 26–53, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Bernard G, Shevell MI. Channelopathies: a review. Pediatr Neurol 38: 73–85, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Bichet D, Cornet V, Geib S, Carlier E, Volsen S, Hoshi T, Mori Y, De Waard M. The I–II loop of the Ca2+ channel alpha1 subunit contains an endoplasmic reticulum retention signal antagonized by the beta subunit. Neuron 25: 177–190, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Bilotta J, Saszik S. The zebrafish as a model visual system. Int J Dev Neurosci 19: 621–629, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Bogdanov Y, Brice NL, Canti C, Page KM, Li M, Volsen SG, Dolphin AC. Acidic motif responsible for plasma membrane association of the voltage-dependent calcium channel beta1b subunit. Eur J Neurosci 12: 894–902, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Brice NL, Berrow NS, Campbell V, Page KM, Brickley K, Tedder I, Dolphin AC. Importance of the different beta subunits in the membrane expression of the alpha1A and alpha2 calcium channel subunits: studies using a depolarization-sensitive alpha1A antibody. Eur J Neurosci 9: 749–759, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Brice NL, Dolphin AC. Differential plasma membrane targeting of voltage-dependent calcium channel subunits expressed in a polarized epithelial cell line. J Physiol 515: 685–694, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burgess DL, Jones JM, Meisler MH, Noebels JL. Mutation of the Ca2+ channel beta subunit gene Cchb4 is associated with ataxia and seizures in the lethargic (lh) mouse. Cell 88: 385–392, 1997. [DOI] [PubMed] [Google Scholar]
- 21.Burns CG, Milan DJ, Grande EJ, Rottbauer W, MacRae CA, Fishman MC. High-throughput assay for small molecules that modulate zebrafish embryonic heart rate. Nat Chem Biol 1: 263–264, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Chen YH, Li MH, Zhang Y, He LL, Yamada Y, Fitzmaurice A, Shen Y, Zhang H, Tong L, Yang J. Structural basis of the alpha1-beta subunit interaction of voltage-gated Ca2+ channels. Nature 429: 675–680, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Chin H, Kwon OJ, Jung HH, Kim DS, Kozak CA. Genetic mapping of the mouse genes encoding the voltage-sensitive calcium channel subunits. Genomics 28: 592–595, 1995. [DOI] [PubMed] [Google Scholar]
- 24.Chu PJ, Larsen JK, Chen CC, Best PM. Distribution and relative expression levels of calcium channel beta subunits within the chambers of the rat heart. J Mol Cell Cardiol 36: 423–434, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Colecraft HM, Alseikhan B, Takahashi SX, Chaudhuri D, Mittman S, Yegnasubramanian V, Alvania RS, Johns DC, Marban E, Yue DT. Novel functional properties of Ca(2+) channel beta subunits revealed by their expression in adult rat heart cells. J Physiol 541: 435–452, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cork RJ, Namkung Y, Shin HS, Mize RR. Development of the visual pathway is disrupted in mice with a targeted disruption of the calcium channel beta(3)-subunit gene. J Comp Neurol 440: 177–191, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Dalton S, Takahashi SX, Miriyala J, Colecraft HM. A single CaVbeta can reconstitute both trafficking and macroscopic conductance of voltage-dependent calcium channels. J Physiol 567: 757–769, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ebert A, McAnelly C, Srinivasan A, Mueller R, Garrity D, Garrity D. The calcium channel β2 (CACNB2) subunit repertoire in teleosts. BMC Mol Biol 9: 38, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ebert AM, McAnelly CA, Srinivasan A, Linker JL, Horne WA, Garrity DM. Ca2+ channel-independent requirement for MAGUK family CACNB4 genes in initiation of zebrafish epiboly. Proc Natl Acad Sci USA 105: 198–203, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Escayg A, De Waard M, Lee DD, Bichet D, Wolf P, Mayer T, Johnston J, Baloh R, Sander T, Meisler MH. Coding and noncoding variation of the human calcium-channel beta4-subunit gene CACNB4 in patients with idiopathic generalized epilepsy and episodic ataxia. Am J Hum Genet 66: 1531–1539, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Escayg A, Jones JM, Kearney JA, Hitchcock PF, Meisler MH. Calcium channel beta 4 (CACNB4): human ortholog of the mouse epilepsy gene lethargic. Genomics 50: 14–22, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Force A, Lynch M, Pickett FB, Amores A, Yan YL, Postlethwait J. Preservation of duplicate genes by complementary, degenerative mutations. Genetics 151: 1531–1545, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gates MA, Kim L, Egan ES, Cardozo T, Sirotkin HI, Dougan ST, Lashkari D, Abagyan R, Schier AF, Talbot WS. A genetic linkage map for zebrafish: comparative analysis and localization of genes and expressed sequences. Genome Res 9: 334–347, 1999. [PubMed] [Google Scholar]
- 34.Gonzalez-Gutierrez G, Miranda-Laferte E, Neely A, Hidalgo P. The SRC homology 3 domain of the beta-subunit of voltage-gated calcium channels promotes endocytosis via dynamin interaction. J Biol Chem 282: 2156–2162, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Haase H, Pfitzmaier B, McEnery MW, Morano I. Expression of Ca(2+) channel subunits during cardiac ontogeny in mice and rats: identification of fetal alpha(1C) and beta subunit isoforms. J Cell Biochem 76: 695–703, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Helton TD, Kojetin DJ, Cavanagh J, Horne WA. Alternative splicing of a beta4 subunit proline-rich motif regulates voltage-dependent gating and toxin block of Cav2.1 Ca2+ channels. J Neurosci 22: 9331–9339, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hibino H, Pironkova R, Onwumere O, Rousset M, Charnet P, Hudspeth AJ, Lesage F. Direct interaction with a nuclear protein and regulation of gene silencing by a variant of the Ca2+-channel beta 4 subunit. Proc Natl Acad Sci USA 100: 307–312, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hidalgo P, Neely A. Multiplicity of protein interactions and functions of the voltage-gated calcium channel beta-subunit. Cell Calcium 42: 389–396, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Hong X, Scofield DG, Lynch M. Intron size, abundance, and distribution within untranslated regions of genes. Mol Biol Evol 23: 2392–2404, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Hsueh YP The role of the MAGUK protein CASK in neural development and synaptic function. Curr Med Chem 13: 1915–1927, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Hullin R, Khan IF, Wirtz S, Mohacsi P, Varadi G, Schwartz A, Herzig S. Cardiac L-type calcium channel beta-subunits expressed in human heart have differential effects on single channel characteristics. J Biol Chem 278: 21623–21630, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Hurley IA, Mueller RL, Dunn KA, Schmidt EJ, Friedman M, Ho RK, Prince VE, Yang Z, Thomas MG, Coates MI. A new time-scale for ray-finned fish evolution. Proc Biol Sci 274: 489–498, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kimmel C, Ballard W, Kimmel S, Ullmann B, Schilling T. Stages of embryonic development of the zebrafish. Dev Dyn 203: 253–310, 1995. [DOI] [PubMed] [Google Scholar]
- 44.Le Hir H, Nott A, Moore MJ. How introns influence and enhance eukaryotic gene expression. Trends Biochem Sci 28: 215–220, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Ludwig A, Flockerzi V, Hofmann F. Regional expression and cellular localization of the alpha1 and beta subunit of high voltage-activated calcium channels in rat brain. J Neurosci 17: 1339–1349, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marmorstein LY, Wu J, McLaughlin P, Yocom J, Karl MO, Neussert R, Wimmers S, Stanton JB, Gregg RG, Strauss O, Peachey NS, Marmorstein AD. The light peak of the electroretinogram is dependent on voltage-gated calcium channels and antagonized by bestrophin (best-1). J Gen Physiol 127: 577–589, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McEnery MW, Vance CL, Begg CM, Lee WL, Choi Y, Dubel SJ. Differential expression and association of calcium channel subunits in development and disease. J Bioenerg Biomembr 30: 409–418, 1998. [DOI] [PubMed] [Google Scholar]
- 48.Nott A, Meislin SH, Moore MJ. A quantitative analysis of intron effects on mammalian gene expression. RNA 9: 607–617, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohno S Evolution by Gene Duplication. Heidelberg, Germany: Springer Verlag, 1970.
- 50.Ohtsuka Y, Okamura Y. Voltage-dependent calcium influx mediates maturation of myofibril arrangement in ascidian larval muscle. Dev Biol 301: 361–373, 2007. [DOI] [PubMed] [Google Scholar]
- 51.Perez-Reyes E, Castellano A, Kim HS, Bertrand P, Baggstrom E, Lacerda AE, Wei XY, Birnbaumer L. Cloning and expression of a cardiac/brain beta subunit of the L-type calcium channel. J Biol Chem 267: 1792–1797, 1992. [PubMed] [Google Scholar]
- 52.Postlethwait J, Amores A, Cresko W, Singer A, Yan YL. Subfunction partitioning, the teleost radiation and the annotation of the human genome. Trends Genet 20: 481–490, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Postlethwait JH, Woods IG, Ngo-Hazelett P, Yan YL, Kelly PD, Chu F, Huang H, Hill-Force A, Talbot WS. Zebrafish comparative genomics and the origins of vertebrate chromosomes. Genome Res 10: 1890–1902, 2000. [DOI] [PubMed] [Google Scholar]
- 54.Pragnell M, De Waard M, Mori Y, Tanabe T, Snutch TP, Campbell KP. Calcium channel beta-subunit binds to a conserved motif in the I-II cytoplasmic linker of the alpha 1-subunit. Nature 368: 67–70, 1994. [DOI] [PubMed] [Google Scholar]
- 55.Prince V The Hox Paradox: more complex(es) than imagined. Dev Biol 249: 1–15, 2002. [DOI] [PubMed] [Google Scholar]
- 56.Rousset M, Cens T, Charnet P. Alone at last! New functions for Ca2+ channel beta subunits? Sci STKE 2005: pe11, 2005. [DOI] [PubMed]
- 57.Sakharkar MK, Chow VT, Kangueane P. Distributions of exons and introns in the human genome. In Silico Biol 4: 387–393, 2004. [PubMed] [Google Scholar]
- 58.Schmitt EA, Dowling JE. Early retinal development in the zebrafish, Danio rerio: light and electron microscopic analyses. J Comp Neurol 404: 515–536, 1999. [PubMed] [Google Scholar]
- 59.Semon M, Wolfe KH. Reciprocal gene loss between Tetraodon and zebrafish after whole genome duplication in their ancestor. Trends Genet 23: 108–112, 2007. [DOI] [PubMed] [Google Scholar]
- 60.Sutherland RJ The dorsal diencephalic conduction system: a review of the anatomy and functions of the habenular complex. Neurosci Biobehav Rev 6: 1–13, 1982. [DOI] [PubMed] [Google Scholar]
- 61.Takahashi SX, Mittman S, Colecraft HM. Distinctive modulatory effects of five human auxiliary beta2 subunit splice variants on L-type calcium channel gating. Biophys J 84: 3007–3021, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tanaka O, Sakagami H, Kondo H. Localization of mRNAs of voltage-dependent Ca(2+)-channels: four subtypes of alpha 1- and beta-subunits in developing and mature rat brain. Brain Res Mol Brain Res 30: 1–16, 1995. [DOI] [PubMed] [Google Scholar]
- 63.Tareilus E, Roux M, Qin N, Olcese R, Zhou J, Stefani E, Birnbaumer L. A Xenopus oocyte beta subunit: evidence for a role in the assembly/expression of voltage-gated calcium channels that is separate from its role as a regulatory subunit. Proc Natl Acad Sci USA 94: 1703–1708, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Taviaux S, Williams ME, Harpold MM, Nargeot J, Lory P. Assignment of human genes for beta 2 and beta 4 subunits of voltage-dependent Ca2+ channels to chromosomes 10p12 and 2q22-q23. Hum Genet 100: 151–154, 1997. [DOI] [PubMed] [Google Scholar]
- 65.Taylor JS, Raes J. Duplication and divergence: the evolution of new genes and old ideas. Annu Rev Genet 38: 615–643, 2004. [DOI] [PubMed] [Google Scholar]
- 65a.Thisse B, Thisse C. Fast release clones: a high throughout expression analysis. ZFIN Direct Data Submission (http://zfin.org).
- 66.Thisse C, Thisse B, Schilling TF, Postlethwait JH. Structure of the zebrafish snail1 gene and its expression in wild-type, spadetail and no tail mutant embryos. Development 119: 1203–1215, 1993. [DOI] [PubMed] [Google Scholar]
- 67.Van Petegem F, Clark KA, Chatelain FC, Minor DL Jr. Structure of a complex between a voltage-gated calcium channel beta-subunit and an alpha-subunit domain. Nature 429: 671–675, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vance CL, Begg CM, Lee WL, Haase H, Copeland TD, McEnery MW. Differential expression and association of calcium channel alpha1B and beta subunits during rat brain ontogeny. J Biol Chem 273: 14495–14502, 1998. [DOI] [PubMed] [Google Scholar]
- 69.Vendel AC, Rithner CD, Lyons BA, Horne WA. Solution structure of the N-terminal A domain of the human voltage-gated Ca2+ channel beta4a subunit. Protein Sci 15: 378–383, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Volff JN Genome evolution and biodiversity in teleost fish. Heredity 94: 280–294, 2005. [DOI] [PubMed] [Google Scholar]
- 71.Weissgerber P, Held B, Bloch W, Kaestner L, Chien KR, Fleischmann BK, Lipp P, Flockerzi V, Freichel M. Reduced cardiac L-type Ca2+ current in Cav(beta)2−/− embryos impairs cardiac development and contraction with secondary defects in vascular maturation. Circ Res 99: 749–757, 2006. [DOI] [PubMed] [Google Scholar]
- 72.Yu AS, Hebert SC, Lee SL, Brenner BM, Lytton J. Identification and localization of renal Na+−Ca2+ exchanger by polymerase chain reaction. Am J Physiol Renal Fluid Electrolyte Physiol 263: F680–F685, 1992. [DOI] [PubMed] [Google Scholar]
- 73.Zhou W, Saint-Amant L, Hirata H, Cui WW, Sprague SM, Kuwada JY. Nonsense mutations in the dihydropyridine receptor beta1 gene, CACNB1, paralyze zebrafish relaxed mutants. Cell Calcium 39: 227–236, 2006. [DOI] [PubMed] [Google Scholar]