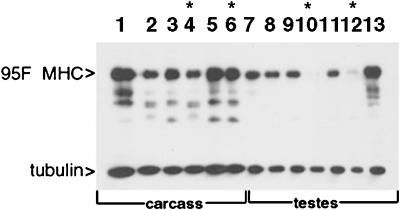
Figure 4.
Western analysis of 95F myosin mutants. Protein was prepared either from carcass (lanes 1–6) or testes (lanes 7–13) of animals of the genotypes indicated. The top half of the blot was probed with anti-95F myosin monoclonal antibody (3C7), and the bottom half of the blot was probed with anti-tubulin monoclonal antibody. Lane 1, wild type; lane 2, Df(3R)S87-5/TM6; lane 3, jarmmw14/TM6, lane 4, jarmmw14/Df(3R)S87-5; lane 5, jar1/TM3; lane 6, jar1/jar1; lane 7, wild type; lane 8, Df(3R)S87-5/TM6; lane 9, jarmmw14/TM6, lane 10, jarmmw14/Df(3R)S87-5; lane 11, jar1/TM3; lane 12, jar1/jar1; lane 13, P[95F MHC]; jarmmw14/Df(3R)S87-5. Asterisks indicate the lanes that show protein from mutant animals. 95F myosin is present in all carcass lanes (lanes 1–6) but is absent in jarmmw14/Df(3R)S87-5 testes (lane 10) and significantly reduced in jar1/jar1 testes (lane 12). The P[95F MHC]-containing animals exhibit high levels of expression in testes in the mutant background, jarmmw14/Df(3R)S87-5 (lane 13). The bands present beneath the 140-kDa 95F myosin band are proteolyzed forms of 95F myosin also recognized by mAb 3C7. The tubulin staining serves as a loading control.