Figure 6. Arp2/3 slows down shell growth by tethering the actin network to the bead surface.
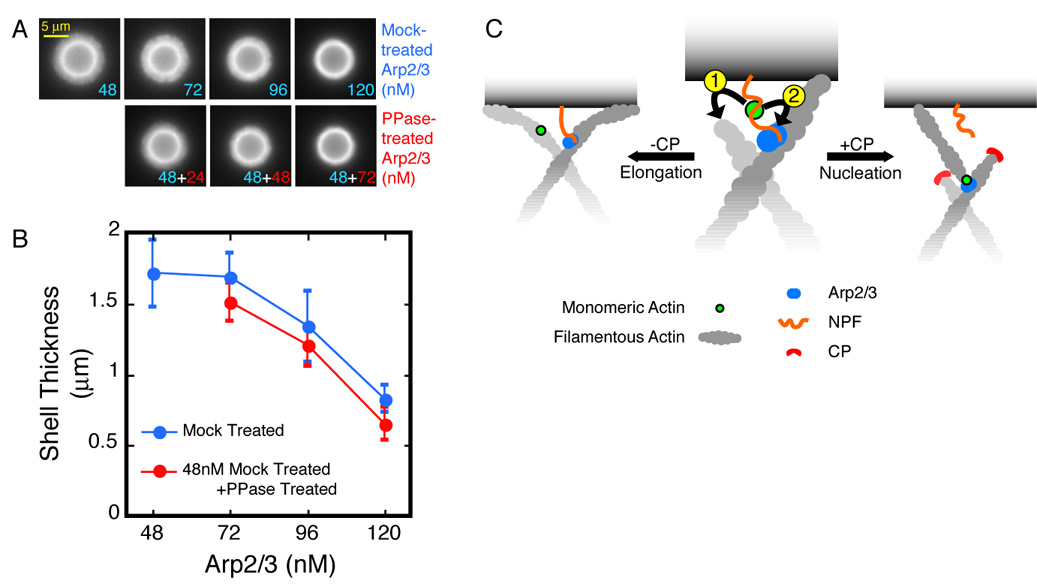
A. Dephosphorylated Arp2/3 complex slows down shell growth as effectively as the nucleation-competent complex. Conditions: 5.8 µm ActA-coated beads mixed with 3 µM actin (5% TMR-labeled), 112 nM CP, and Arp2/3 as indicated. Actin assembly was arrested 60 seconds after mixing.
B. Shell sizes for the conditions in (A). Error bars represent standard deviation (average n=20).
C. Monomer Gating: NPF-bound actin monomers can either associate with nearby barbed ends (1) and contribute to filament elongation, or they can participate in dendritic nucleation (2). Elongation is kinetically favored over nucleation, which requires the stable association of four protein partners—the mother filament, Arp/3, NPF, and the actin monomer—through a slow activation step. CP, by eliminating the competition from barbed ends, gates the biochemical path of actin monomers toward nucleation.
