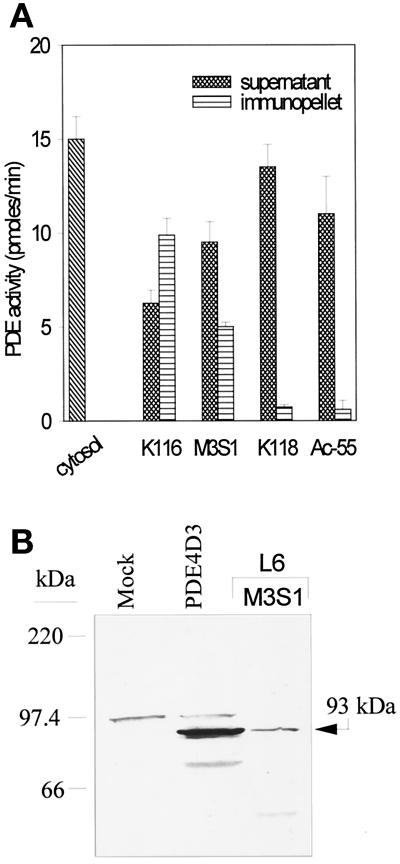
Figure 5.
Characterization of PDE4 proteins expressed in L6-C5 cells. (A) Selective immunoprecipitation of PDE4D. L6-C5 cells were homogenized in hypotonic homogenization buffer as detailed in MATERIALS AND METHODS. Aliquots of the soluble fraction obtained after centrifugation for 15 min at 15,000 × g were immunoprecipitated with the different antibodies. PDE activity was assayed in the starting cytosolic fraction, in the supernatants, and in the immunopellets. The experiment was performed three times with similar results. (B) Western blot analysis of PDE4 proteins expressed in L6-C5 cells. Immunopellet of the L6-C5 cell soluble fraction precipitated with M3S1 antibody was subjected to Western blot analysis, using K116 antiserum for the immunodetection of blots. For comparison, recombinant PDE4D3 protein obtained in 293 cells as previously described (Sette et al., 1994c) was analyzed, in parallel with an extract of mock-transfected 293 cells.