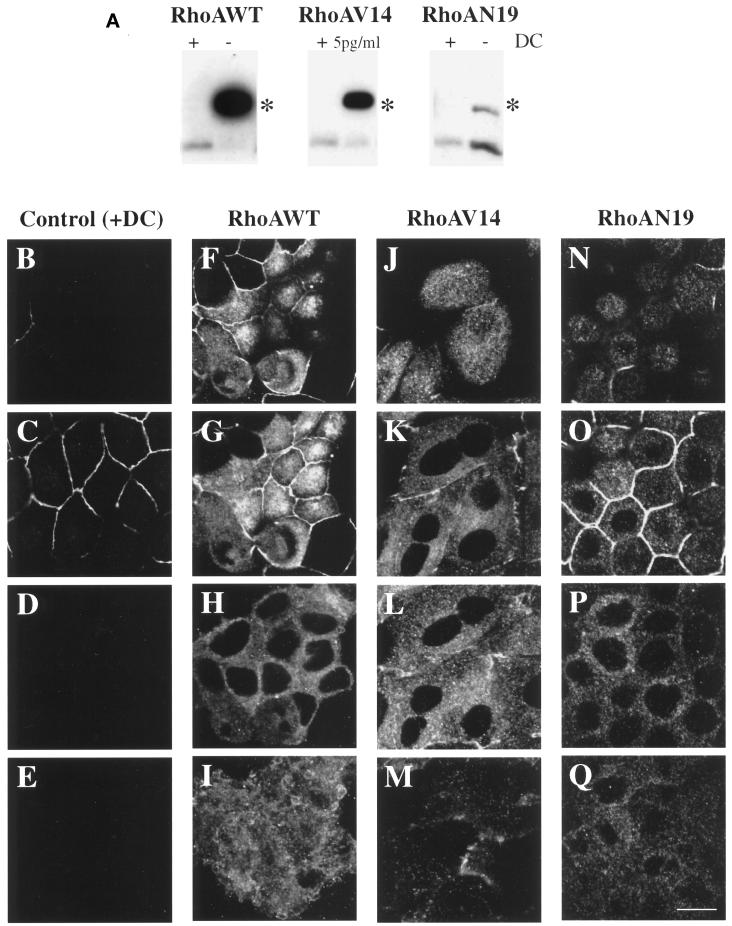
Figure 1.
Inducible expression and distribution of myc-tagged RhoAWT, RhoAV14, and RhoAN19 in polarized MDCK cells. (A) RhoAWT, RhoAV14, and RhoAN19 cells were plated at low density in medium containing 0–5 pg/ml DC (− or 5pg/ml) or containing 20 ng/ml DC (+), incubated for 36–48 h, and then plated on Transwell filter supports (with or without DC). After 18 h, the filter-grown cells were solubilized in SDS lysis buffer, 10 μg of lysate was resolved by PAGE, and Western blots were probed with an anti-RhoA mAb to detect induction of the myc-tagged mutant proteins as well as endogenous RhoA. Asterisks indicate that the addition of the myc tag to RhoAWT, RhoAV14, and RhoAN17 causes these proteins to migrate slower than endogenous RhoA. (B–Q) Distribution of ZO-1 and myc-taggedproteins in RhoAV14 cells grown in the presence of 20 ng/ml DC (B–E), RhoAWT cells grown in the absence of DC (F–I), RhoAV14 cells grown in the presence of 5 pg/ml DC (J–M), or RhoAN19 cells grown in the absence of DC (N–Q). Cells were fixed with paraformaldehyde and stained with antibodies that recognize the tight junction protein ZO-1 or the myc tag, and emission from FITC-conjugated secondary antibodies was captured with the use of a scanning laser confocal microscope. Shown are optical sections from the base of the cells (E, I, M, and Q), along the lateral surface of the cells (D, H, L, and P), at the level of the tight junctions (C, G, K, and O), and at the very apex of the cells (B, F, J, and N). The tight junctions are the thin, brightly stained lines that surround each cell. IgA was internalized at the basolateral pole of these cells, but staining for this marker is not shown. Bar, 10 μm.