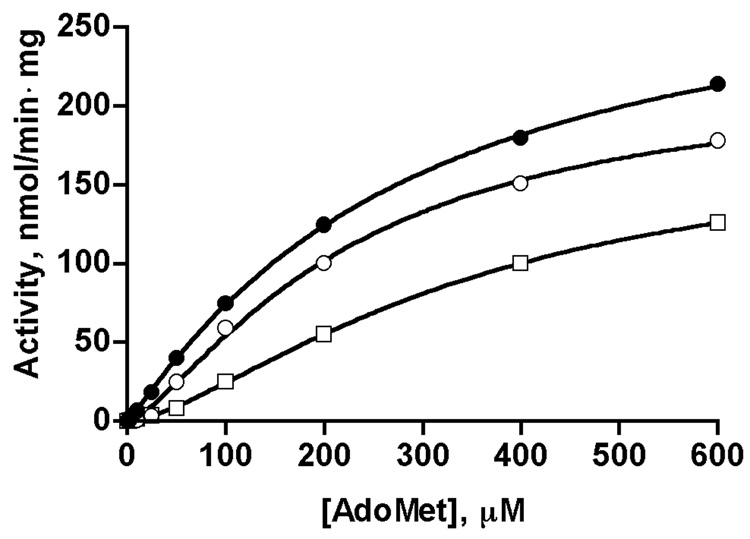
Fig. 4. Cooperativity of dependence of reaction velocity of rat liver GNMT on AdoMet concentrations in presence of 5-CH3-THF-pentaglutamate.
Dependence of velocity on AdoMet concentrations is shown for GNMT apo-protein (closed circles, taken from Fig.1), GNMT-5-CH3-THF-Glu5 complex after incubation with 1 mM 5-CH3-THF-Glu5 followed by passage through Spin-Columns to remove excess folate, as described in the text (open circles), and in the presence of 1 µM 5-CH3-THF-Glu5 in the reaction mixture (open squares). Data fitted with the Hill equation.