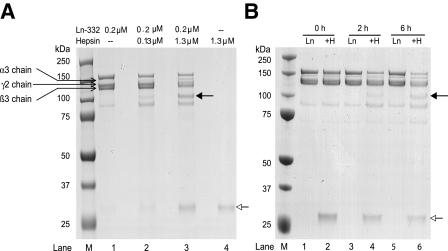
FIGURE 1.
SDS-PAGE analysis of hepsin cleavage of purified rat laminin-332. A, purified rat Ln-332 (0.2 μm) was incubated alone or with the recombinant extracellular domain of hepsin for 1.5 h at 37 °C, electrophoresed on 4–12% gradient gel under reducing conditions, and stained with Coomassie Blue. After incubation of Ln-332 alone, the gel included bands identified as the α3 (190 kDa), β3 (145 kDa), and γ2 (155 and 80 kDa) chains (lane 1). However, upon incubation of Ln-332 with 0.13 or 1.3 μm hepsin (lanes 2 and 3, respectively), an additional ∼100 kDa band was seen (indicated by a solid arrow), indicating a cleavage event. Those lanes including hepsin treatment (lanes 2–4) also produced an ∼30 kDa band, which represents the protease domain of hepsin (indicated by an open arrow). B, after incubation of Ln-332 alone at various time points (0, 2, and 6 h), the same uncleaved Ln-332 chains are visible. However, co-incubation of Ln-332 (0.2 μm) with hepsin (1.3 μm) resulted in the generation of a new band (∼100 kDa, indicated by a closed arrow), again indicating cleavage. Again, the ∼30 kDa band corresponds to the hepsin protease domain (indicated by an open arrow; lanes 2, 4, and 6).