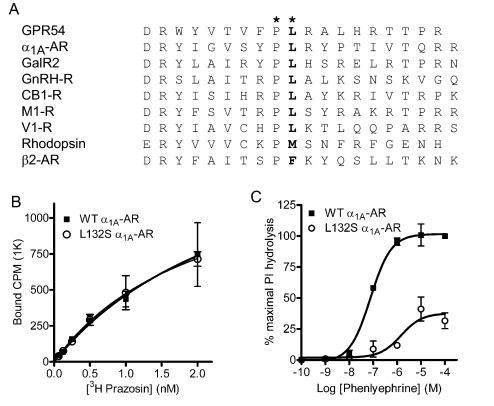
FIGURE 3.
The highly conserved Leu148 of GPR54 is a critical determinant of effective G-protein coupling for diverse Class A GPCRs. A, alignment of IL2 of a number of Class A GPCRs highlights the conservation of proline and leucine residues (*) downstream of the DRY motif that initiates IL2. B, [3H]prazosin saturation radioligand binding shows that mutation of leucine 132 to serine does not alter the ligand-binding properties of the α1A-AR. Results in B show the means ± S.E. of three experiments performed in triplicate. C, the L132S mutation markedly inhibits α1A-AR functional coupling, as measured by phenylephrine-stimulated PI hydrolysis. Results in C are normalized to the response of WT α1A-AR to 100 μm phenylephrine (maximal response) and presented as the means ± S.E. of four experiments performed in triplicate.