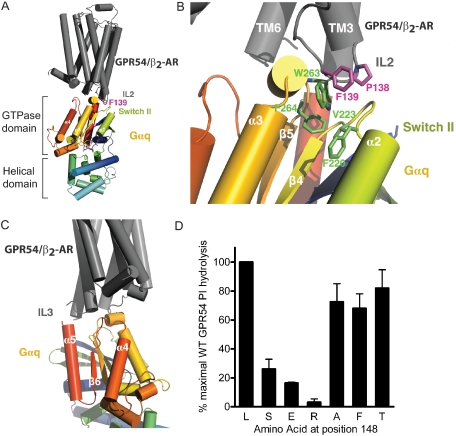
FIGURE 4.
A model to suggest the involvement of IL2 in hydrophobichydrophobic interactions between GPCR and G-protein. A, docking analysis reveals that the IL2 of GPR54/β2-AR comes into close proximity to the GTPase domain of the activated Gαq subunit. B, Pro138 of the β2-AR positions Phe139 so that a productive hydrophobic interaction face is formed with highly conserved Phe220, Val223, Trp263, and Phe264 residues of Gαq. C, the third intracellular loop of GPR54/β2-AR also comes into close contact with the α4/β6 loop of the Gαq subunit. D, mutational analysis of GPR54 demonstrates that the presence of a highly hydrophilic, acidic, or basic amino acid at position 148 inhibits functional coupling, as measured by PI hydrolysis. Data are normalized to the WT response to 1 μm KP-10, which is set as 100%. Results are expressed as the means ± S.E. of four experiments performed in triplicate.