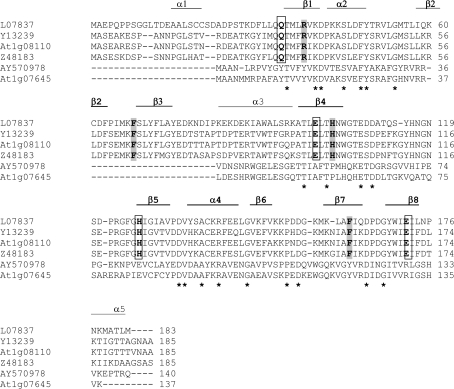
Fig. 1.
ClustalW alignment of amino acid sequences of X. humilis HC205 (AY570978) and the A. thaliana orthologue (At1g07645), with known glyoxalase I genes, including L07837 (Homo sapiens), Y13239 (Brassica juncea), At1g08110 (A. thaliana), and Z48183 (Lycopersicon esculentum). The asterisks represent amino acids that are identical in all of the six sequences. The βαβββ structural repeat determined in the X-ray crystal structure of the human glyoxalase I protein is indicated. This structural repeat is represented by β1α2β2β3β4 in the first domain (α1−β4), and by β5α4β6β7β8 in the second domain (β5−α6) (Cameron et al., 1997). Conserved amino acids that constitute the glutathione binding sites of glyoxalase I are highlighted in grey. Conserved amino acids which form the zinc binding sites are in bold and are boxed.