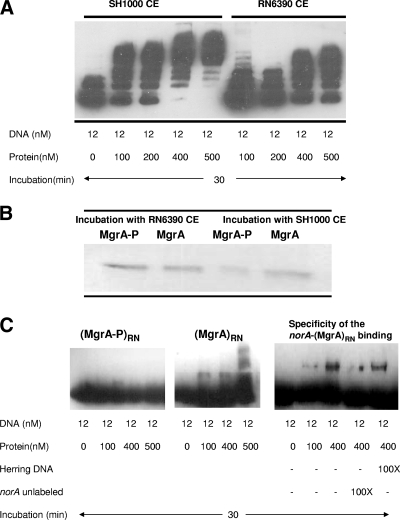
FIG. 2.
A. Gel mobility shift analyses of the interactions of the purified MgrA protein with the biotinylated norA promoter DNA. (A) Protein and DNA were in contact for 30 min at room temperature, followed by electrophoresis through a 5% acrylamide gel. CE, crude extract. (B) SDS-PAGE analysis of histidine-tagged MgrA purified by Ni affinity chromatography after incubation with crude cell extracts. The column was washed with buffers containing 10 and 60 mM imidazole, and the purified protein was eluted with buffer containing 300 mM imidazole. The phosphorylated protein was then purified using a phosphocolumn (Qiagen) following the manufacturer's protocol. The presence of phospho-MgrA was confirmed by Western blotting using AntiSerine antibody (Qiagen) (data not shown). CE, crude extract. (C) Gel mobility shift analyses of the interactions of the purified MgrA and phospho-MgrA proteins with the biotinylated promoter of norA. The two forms of MgrA were purified after incubation with RN6390 crude extract. Unlabeled norA fragment and herring sperm DNA were used to assess the specificity of binding. Protein and DNA concentrations and ratios of unlabeled to labeled DNA used in this assay are indicated in the table below the figures. RN, RN6390.