Fig. 3.
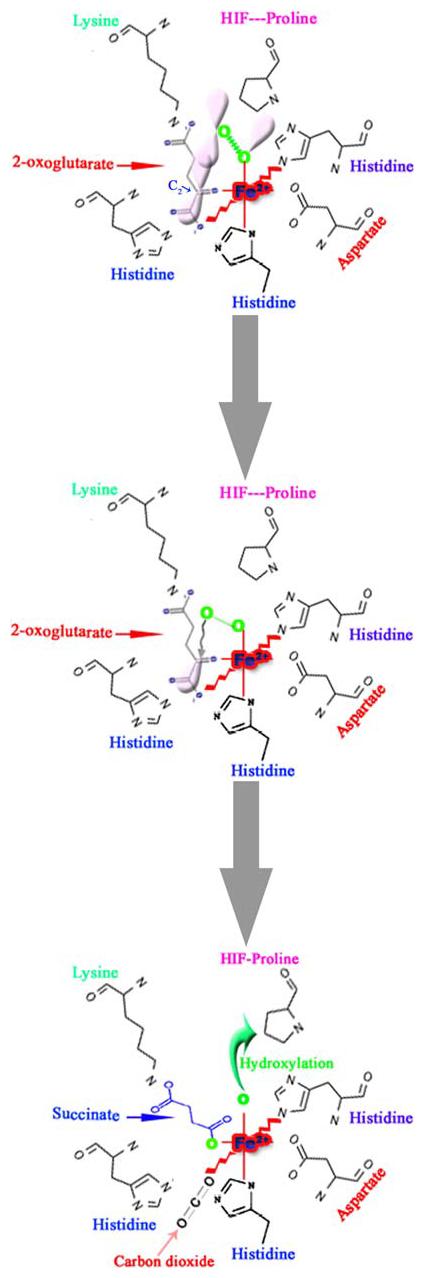
Schematic representation of the first half-reaction of prolyl 4-hydroxylase that takes place within the coordination sphere of the catalytic site iron (Fe2+) and the critical residues at the co-substrate binding sites. The Fe2+ is coordinated with the enzyme by two histidines (His) and an aspartate (Asp). The 2-oxoglutarate-binding site can be divided into two main subsites; subsite I consists of a Lysine residue (Lys), which ionically binds the C5 carboxyl group of 2-oxoglutarate, while subsite II consists of two cis-positioned equatorial coordination sites of enzyme-bound Fe2+ and is chelated by the C1 carboxyl and C2 oxo functions. Molecular oxygen is assumed to be bound end-on in an axial position, producing a dioxygen unit. (A) One of the electron-rich orbitals of the dioxygen is directed to the electron-depleted orbital at the C2 of the 2-oxoglutarate bound to the iron. (B) Nucleophilic attack on C2 generates a tetrahedral intermediate, with loss of the double bond in the dioxygen unit and of double bond characteristics in the oxo-acid moiety. (C) Elimination of CO2 coincides with the formation of succinate and a ferryl ion, which hydroxylates a proline residue in the peptide substrate in the second half-reaction. An additional amino acid in the vicinity of the catalytic triad is an important residue histidine, probably being involved in both coordination of the C1 carboxyl group of 2-oxoglutarate to Fe2+ and cleavage of the tetrahedral ferryl intermediate (Modified from Kivirikko et al. [43])
