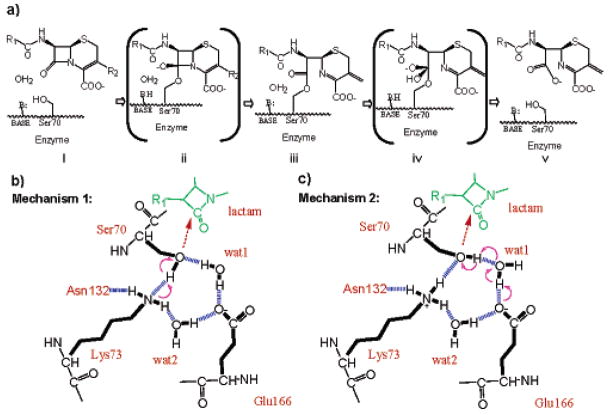
Figure 1.
Possible acylation mechanisms of class A β-lactamases. (a) The overall reaction pathway for β-lactam hydrolysis. Beginning with a ground-state Michaelis complex (i), catalysis proceeds through a high-energy acylation transition state (ii) to an acyl-enzyme intermediate (iii). These steps constitute the acylation half of the reaction. Deacylation then proceeds through a high-energy deacylation transition state (iv) and a postcovalent product complex (v), after which the product leaves. (b) Acylation mechanism 1: a neutral Lys73 as the general base. Hydrogen bonds are indicated by blue dashed lines and electron transfer by purple arrows. (c) Acylation mechanism 2: Glu166 as the general base, through the catalytic water (wat1). Lys73 is positively charged in this model.