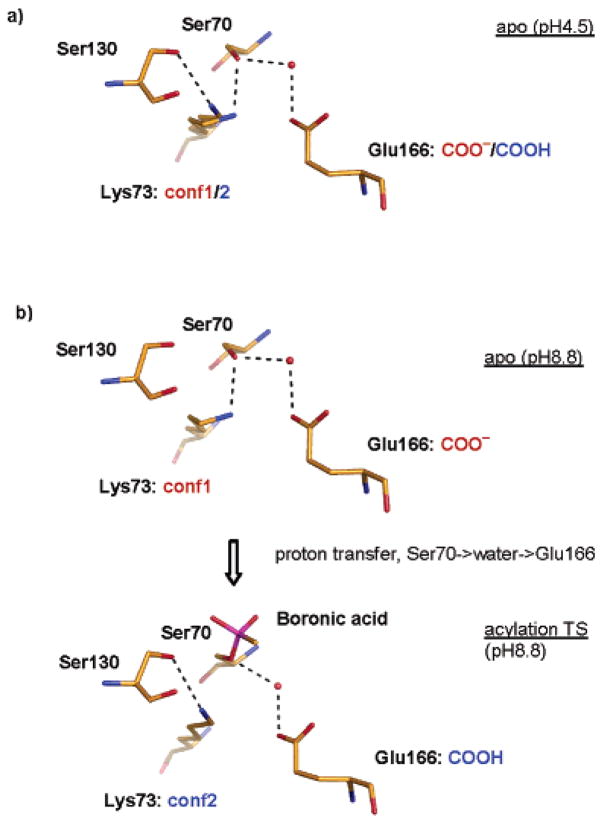
Figure 4.
Conformation change during acylation catalysis. (a) The apo active site configuration observed at pH 4.5. The two conformations observed for Lys73 may correspond to the dual protonation states of Glu166. The putative partial protonation of Glu166 is indicated by the anionic (red) and neutral (blue) text. (b) Lys73 switches conformation from 1 in the apo-active site to 2 in the acylation transition-state analogue structure. Only the tetrahedral boronic acid group (magenta) is shown for the acylation transition-state analogue complex; the rest of the transition-state analogue is pointed outside the plane of the paper.