Abstract
Oxidation of tryptophan to kynurenine and 3-hydroxykynurenine (3-HK) is the major catabolic pathway in mosquitoes. However, 3-HK is oxidized easily under physiological conditions, resulting in the production of reactive radical species. To overcome this problem, mosquitoes have developed an efficient mechanism to prevent 3-HK from accumulating by converting this chemically reactive compound to the chemically stable xanthurenic acid. Interestingly, 3-HK is a precursor for the production of compound eye pigments during the pupal and early adult stages; consequently, mosquitoes need to preserve and transport 3-HK for compound eye pigmentation in pupae and adults. This review summarizes the tryptophan oxidation pathway, compares and contrasts the mosquito tryptophan oxidation pathway with other model species, and discusses possible driving forces leading to the functional adaptation and evolution of enzymes involved in the mosquito tryptophan oxidation pathway.
Keywords: Xanthurenic acid, Mosquito, 3-Hydroxykynurenine, Kynurenine 3-monooxygenase, 3-Hydroxykynurenine transaminase
1. Introduction
Kynurenine and 3-hydroxykynurenine (3-HK) are intermediates in the tryptophan oxidation pathway (Fig. 1). In mammals, kynurenine is the immediate precursor in the pathway leading to the formation of kynurenic acid that serves as a broad-spectrum antagonist at ionotropic excitatory amino acid receptors (NMDA receptors) and protects the central nervous system (CNS) from being overstimulated by excitatory cytotoxins (Stone, 2000). Therefore, there has been extensive research investigating the biochemical pathway leading to the formation of kynurenic acid and the consequences caused by kynurenic acid deficiency (Schwarcz, 1993; Moroni, 1999;Stone, 2001a, c, b). Kynurenine can be oxidized by kynurenine monooxygenase (KMO, EC 1.14.13.9) to form 3-HK. Although 3-HK is a natural metabolite, it is oxidized easily under physiological conditions, stimulating the production of reactive oxygen species (Okuda et al., 1996, 1998; Wei et al., 2000). For example, 3-HK can induce apoptosis of neuron cells at micromolar concentrations (Wei et al., 2000). Injection of tryptophan metabolites into adult flies caused severe motor dysfunction (Cerstiaens et al., 2003). Earlier studies have also shown that 3-HK content was quite different in insects at different developmental stages (Linzen, 1974). To maintain physiological conditions, it is essential to prevent the accumulation of this reactive compound. In mammals, both kynurenine and 3-HK can be hydrolyzed by kynureninase (EC 3.7.1.3) to anthranilic acid and 3-hydroxyanthranilic acid and the latter two compounds can be either completely oxidized to CO2 and H2O through a complicated biochemical pathway or used to synthesize NAD(P)+ (Stone, 1993). Although there have been a number of reports discussing the toxicity of 3-HK in mammals (Okuda et al., 1996, 1998; Wei et al., 2000), 3-HK, produced under normal physiological conditions, does not seem to cause any problems in mammals as it can be hydrolyzed and further oxidized via the glutaryl-CoA pathway.
Fig. 1.
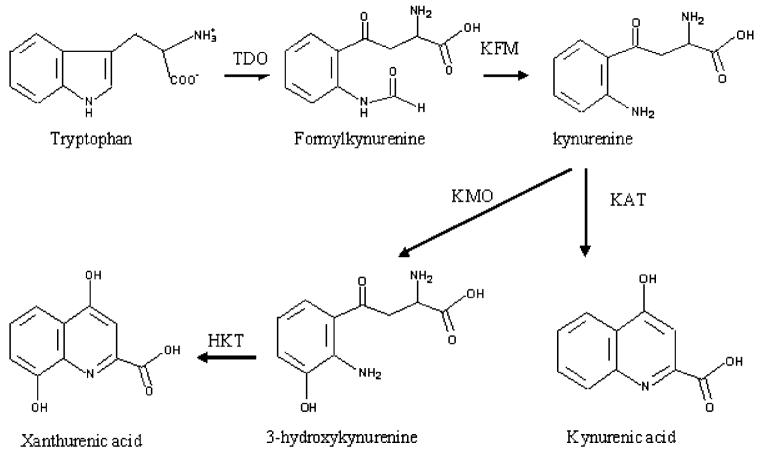
Biochemical pathway of tryptophan to xanthurenic acid in mosquitoes. TDO, tryptophan dioxygenase; KFM, kynurenine formamidase; KMO, kynurenine monooxygenase; KAT, kynurenine aminotransferase; HKT, 3-hydroxykynurenine transaminase.
In mosquitoes (likely other insects as well), oxidation of tryptophan to kynurenine and then to 3-HK is a major branch pathway of tryptophan metabolism. However, because mosquitoes do not have kynureninase, the hydro-lysis pathway of 3-HK is not available. Consequently, mosquitoes must deal with 3-HK in a different manner. Our study investigating the tryptophan oxidation pathway in Aedes aegypti indicated that mosquitoes have developed an efficient strategy to prevent the accumulation of 3-HK by converting the chemically reactive 3-HK to the chemically stable xanthurenic acid (XA) via transaminase-mediated reactions. For example, the concentration of XA is many folds higher than that of 3-HK in the supernatant of mosquito larval homogenate (Li and Li, 1997; Li et al., 1999) (Li, J., unpublished data). As there have been no reports indicating any toxic effect of XA to living organisms, we proposed that the transamination of the chemically reactive 3-HK to the chemically stable XA serves as the mechanism by which mosquitoes detoxify 3-HK (Li and Li, 1997, 1998;Han et al., 2002).
Recently, the 3-HK to XA pathway has attracted increased attention because it has been found that XA induces the exflagellation of Plasmodium microgametocytes (Billker et al., 1998; Garcia et al., 1998), an essential step during sexual reproduction of malaria parasites in mosquitoes. Mosquitoes transmit malaria parasites, dengue fever and West Nile virus, which are major threats to human health and well-being throughout the world. Among them, malaria is considered to be the most prevalent life-threatening disease, with estimates of new cases ranging from 300 million to 660 million cases per year (Snow et al., 2005). The development of insecticide-resistant mosquitoes and drug-resistant parasites demand that new innovative control strategies have to be developed. Therefore, the ability of XA to stimulate Plasmodium development provides a potential malaria control strategy by targeting proteins involved in the 3-HK to XA pathway in mosquitoes (Rossi et al., 2005, 2006).
Interestingly, 3-HK also is the initial precursor for the production of ommochromes that are major eye pigments in mosquitoes. Compound eye development and eye pigmentation occur mainly during the pupal and early adult stages (Beard et al., 1995; Li et al., 1999; Rasgon and Scott, 2004; Sethuraman and O’Brochta, 2005). Consequently, the 3-HK to XA pathway must be partially down-regulated in pupae and adults to allow for the accumulation of some 3-HK that then can be transported to the compound eyes for pigmentation (Li and Li, 1997; Li et al., 1999). In this review, we summarize the tryptophan to XA pathway in living organisms, compare and contrast the same pathway in mosquitoes with that of other organisms, and discuss the functional adaptation and evolution of the proteins involved in the 3-HK to XA pathway in mosquitoes.
2. The tryptophan to XA pathway
XA is a metabolite in the tryptophan oxidation pathway and its formation from tryptophan is a complicated process with a number of enzymes involved in the biochemical pathway. The overall process leading to the formation of XA includes oxidation of tryptophan to formylkynurenine, hydrolysis of formylkynurenine to kynurenine, hydroxylation of kynurenine to 3-HK, transamination of 3-HK to a side chain keto acid intermediate, and intramolecular cyclization of the intermediate to XA (Fig. 1). The chemical process and enzymes involved in this branch pathway of tryptophan metabolism have been studied extensively in mammals. Because metabolites in the tryptophan oxidation pathway influence the color of the compound eyes in insects, there have been a number of earlier reports discussing tryptophan metabolites in relation to insect compound eye development (Howells et al., 1977; Paton and Sullivan, 1978; Summers and Howells, 1978; Akaboshi, 1979; Howells, 1979). However, there have been few studies concerning the enzymes involved in the tryptophan oxidation pathway in insects.
2.1. Tryptophan 2,3-dioxygenase (TDO)
TDO is a heme-containing dioxygenase involved in catalyzing the addition of molecular oxygen (O2) across the 2,3-double bond of the indole ring of tryptophan, leading to the cleavage of the indole ring to form N-formylkynurenine (Tanaka and Knox, 1959; Hayaishi and Nozaki, 1969; Leeds et al., 1993; Dick et al., 2001). It has generally been accepted that the heme prosthetic group of TDO is present as a heme-ferric form (heme-Fe3+) that must be reduced to the heme-ferrous form (heme-Fe2+) prior to mediating tryptophan oxidation (Tanaka and Knox, 1959;Hayaishi and Nozaki, 1969; Ishimura and Hayaishi, 1973;Hitchcock and Katz, 1988). The mechanism leading to TDO activation has attracted a considerable amount of attention. It has been reported that superoxide (•O2-) or hydrogen peroxide (H2O2) or reducing agents (e.g., ascorbate and sodium hydrosulfite) stimulate TDO activity through a reductive activation process (Tanaka and Knox, 1959; Hayaishi and Nozaki, 1969; Brady et al., 1971;Schutz et al., 1972; Ishimura and Hayaishi, 1973; Hitchcock and Katz, 1988). Earlier studies also concluded that tryptophan 2,3-dioxygenase was subject to allosteric regulation and that the enzyme had at least two distinct sites for tryptophan binding, with one as a catalytic site and the other as an allosteric site (Koike et al., 1969; Brady et al., 1971; Schutz et al., 1972; Kobayashi et al., 1989).
The early studies of insect tryptophan oxidation focused on Drosophila TDO which also was called tryptophan pyrrolase (Kaufman, 1962; Marzluf, 1965; Phillips et al., 1967; Burnet and Sang, 1968; Tartof, 1969; Baillie and Chovnick, 1971; Jacobson, 1971; Tobler et al., 1971;Sullivan et al., 1974; Mischke et al., 1975; Moore and Sullivan, 1975; Tobler, 1975). Subsequently, it was verified that the vermilion gene that affects the color of Drosophila compound eyes is Drosophila TDO (Searles and Voelker, 1986; Walker et al., 1986; Searles et al., 1990). After the molecular characterization of the Drosophila TDO (Gen-Bank accession no. AAC24239), the TDO gene from Anopheles gambiae (GenBank accession no. AAC27659) and Tribolium (GenBank accession no. AAL15466) also has been molecularly cloned (Mukabayire et al., 1996; Lorenzen et al., 2002). Presently, a number of insect TDO sequences are available in the genomic databases. However, there have been no in-depth studies dealing with any insect TDO at the protein level, likely due to difficulties in isolating sufficient TDO from insects for critical characterization. Recently, we expressed an Ae. aegypti TDO (GenBank accession no. AF325458) in a baculovirus/insect cell system, and this recombinant TDO was functionally active as shown by its ability to oxidize tryptophan to formylkynurenine (Fig. 2). The ability to purify milligram quantities of functionally active TDO from transfected insect cells should provide the necessary material required for understanding the structure/function relationship of this important enzyme.
Fig. 2.
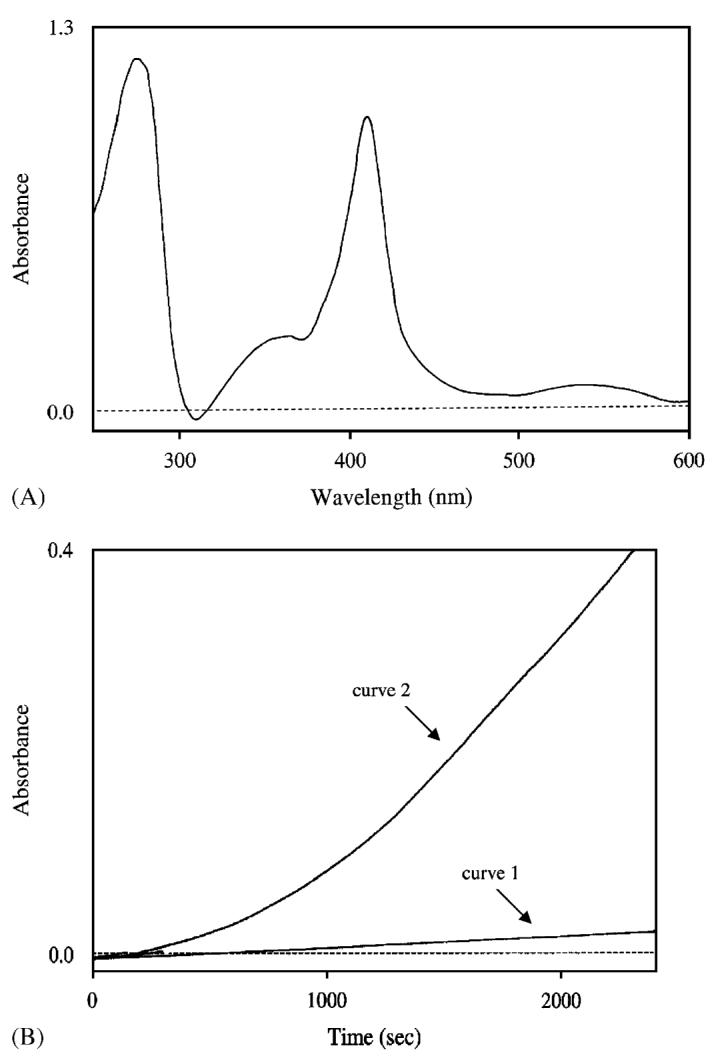
Spectral characteristics and biochemical activity of the recombinant Ae. aegypti tryptophan 2,3-dioxygenase (TDO). (A) Spectral characteristics of the purified recombinant Ae. aegypti TDO (about 1 mg in 1.0 ml of phosphate buffer, pH 7.5). TDO is a heme-containing enzyme. The presence of an absorbance peak with a λmax at 405 nm indicates that the heme prosthetic group is present in the enzyme. (B) Production of formylkynurenine in a TDO and tryptophan reaction mixture. Presence of an initial lag phase in formylkynurenine production in a TDO and tryptophan reaction mixture suggests that Ae. aegypti TDO is present as an inactive form, and addition of tryptophan results in its progressive activation. Curve 1 and curve 2 illustrate absorbance change at 321 nm in tryptophan solutions in the absence and presence of TDO (20 μg/ml reaction mixture), respectively.
2.2. Kynurenine formamidase
Formylkynurenine, formed by TDO-catalyzed tryptophan oxidation, is hydrolyzed to kynurenine (see Fig. 1). Hydrolysis of formylkynurenine proceeds under physiological conditions, but the rate is low. A specific enzyme, which catalyzes the hydrolysis of formylkynurenine, has been shown to be present in a number of mammalian species and is responsible for the rapid conversion of formylkynurenine to kynurenine in vivo (Mehler and Knox, 1950). This enzyme has been termed kynurenine formamidase (EC 3.5.1.9) and the enzyme from adult chicken liver (Bailey and Wagner, 1974), rat liver cytoplasm (Arndt et al., 1973), Drosophila melanogaster (Moore and Sullivan, 1975, 1978), and Streptomyces parvulus (Brown et al., 1986) has been partially purified. A mouse kynurenine formamidase recently has been cloned (GenBank accession no. NP_082103) and characterized (Pabarcus and Casida, 2002). A gene coding for kynurenine formamidase also has been identified from a bacterium (Kurnasov et al., 2003). However, no sequence in the Drosophila and An. gambiae protein databases has been annotated as the kynurenine formamidase. A BLAST search and sequence alignment revealed that the coding sequences with GenBank accession numbers of AAF52391 and EAA03628, respectively, sharing 25% and 35% sequence identity with the mouse formamidase, are likely to be the kynurenine formamidase in Drosophila and An. gambiae, respectively. The true identity of these two proteins, however, must be functionally verified through critical biochemical characterization.
2.3. Kynurenine 3-monooxygenase (KMO)
KMO (EC 1.14.13.9), a flavin-containing enzyme, catalyzes the hydroxylation of kynurenine to 3-HK in the tryptophan oxidation pathway. KMO has a key role in tryptophan catabolism and the synthesis of ommochrome pigments in mosquitoes. Early studies of KMO were concerned primarily with its role in eye pigmentation in some insects, including D. melanogaster (Howells et al., 1977; Paton and Sullivan, 1978; Howells, 1979), Lucilia cuprina (Summers and Howells, 1978) and Musca domestica (Akaboshi, 1979). In D. melanogaster, the cinnabar (cn) gene (GenBank accession no. NP_523651) was shown to encode KMO (Warren et al., 1996) and in a mutant strain of Bombyx mori, the genetic lesion leading to the white-eye phenotype has been identified (Quan et al., 2002). The gene encoding KMO (GenBank accession no. AAO27576) in the yellow fever mosquito, Ae. aegypti, has been named kynurenine hydroxylase (kh) (Cornel et al., 1997), and its mutation results in a white-eye phenotype designated as the khw strain (Bhalla, 1968; Cornel et al., 1997). Recent efforts to genetically manipulate mosquitoes to control mosquito-transmitted diseases have raised considerable interest in the KMO gene, because it can serve as an excellent marker that indicates the successful production of transgenic Ae. aegypti (Coates et al., 1998; Jasinskiene et al., 1998;Sethuraman and O’Brochta, 2005). Sequence analysis of the wild-type and mutant khw cDNAs revealed a deletion of 162 nucleotides in the mutant Ae. aegypti KMO gene near the 3′-end of the deduced coding region and RT-PCR analysis confirmed the transcription of a truncated mRNA in the mutant strain (Han et al., 2003). The in-frame deletion of the KMO gene results in a loss of 54 amino acids and disrupts a major alpha-helix, which probably accounts for the loss of its activity. Further evidence has shown that the Ae. aegypti white-eye (khw) mutant strain (Bhalla, 1968) can be complemented by a wild-type copy of the D. melanogaster cinnabar gene (Cornel et al., 1997), thereby providing genetic evidence that the product encoded by the white-eye gene was KMO. Although the D. melanogaster cinnabar gene complements various white-eye and other mutations in Diptera, it does not function well outside this order (Atkinson et al., 2001). However, the general application of the eye-color genes as broad-spectrum transformation markers in species of Coleoptera has also been discussed (Lorenzen et al., 2002).
The catalytic mechanism of KMO has been suggested as being similar to that of a bacterial p-hydroxybenzoate hydroxylase (Entsch et al., 1976a, b; Breton et al., 2000). Sequence comparison between the Ae. aegypti KMO and Pseudomonas putida hydroxybenzoate hydroxylase (PHBH), an extensively characterized flavin monooxygenase, revealed similarity between the two enzymes. Although Ae. aegypti KMO and P. putida PHBH share only limited overall sequence identity (16%), the Ae. aegypti KMO, like the P. putida PHBH, contains a similar sequence motif xhxhGxxGxxxhxxh(x)8hxhE(D), which is a well-known fingerprint for a dinucleotide binding domain, where x is any residue and h is a hydrophobic residue (Eppink et al., 1997). Moreover, the amino acid sequence, 153DYIAGCDGFHGISR166, which has been reported to play a dual function in both FAD and NAD(P)H binding in hydroxybenzoate hydroxylase, also is highly conserved in the Ae. aegypti KMO (residues 172DLIVGCDGAYSAVR185). Based on these observations, the catalytic mechanism of KMO likely is similar to that of hydroxybenzoate hydroxylase, a prototype for a FAD-dependent monooxygenase. However, some sequence fragments that are highly conserved in KMOs are not present in hydroxybenzoate hydroxylase. For example, the residues 60–68, 225–230, 233–240 and 326–332 in the Ae. aegypti KMO likely play relevant roles in either folding stability or catalysis. KMO has been cloned from several insect species and the biochemical and kinetic parameters of the enzyme have been analyzed for a few of them (Hirai et al., 2002; Quan et al., 2002). For example, recombinant Ae. aegypti KMO showed a high substrate specificity for kynurenine with optimum activity at 40 °C and pH = 7.5. Its Km for kynurenine, NADPH and NADH was 0.89, 0.82 and 5.17, respectively, suggesting that it favors NADPH as a reducing agent. KMO is considered to be an important pharmaceutical target for the development of drugs for neurodegenerative diseases (Pellicciari et al., 2003; Moroni et al., 2005; Samadi et al., 2005); therefore, there is an increasing need to determine its 3D structure.
2.4. Kynurenine aminotransferase
In humans and other mammals, kynurenine aminotransferase (KAT) I and II are the primary enzymes involved in the transamination of 3-HK (Okuno et al., 1991; Guidetti et al., 1997; Han et al., 2004). However, the 3-HK transamination function of KAT was rarely highlighted in the published literature. KAT, as implied by its name, catalyzes the transamination of kynurenine to kynurenic acid, a broad-spectrum antagonist at ionotropic excitatory amino acid receptors (NMDA receptors) that protects the central nervous system from being over-stimulated by excitatory cytotoxins (Stone et al., 1996). Although mammalian KAT efficiently catalyzes both the kynurenine to kynurenic acid pathway and the 3-HK to XA pathway, the emphasis on KAT function has been exclusively on its role in the biosynthesis of kynurenic acid (Moroni, 1999; Stone and Darlington, 2002; Schwarcz, 2004). Initially, it was assumed that a similar KAT was responsible for the 3-HK to XA pathway in Ae. aegypti. The Ae. aegypti KAT (GenBank accession no. AAK97625) was successfully cloned and its deduced sequence shares high sequence identity with mammalian KATs (Fang et al., 2002). Subsequently, an Ae. aegypti recombinant KAT was expressed in insect cells, and biochemical characterization revealed that the mosquito KAT had high activity towards kynurenine, but it showed no activity for 3-HK (Fang et al., 2002; Han and Li, 2004). By following the 3-HK transamination active fractions during chromatographic separation, we eventually purified the 3-HK transaminase (HKT) from Ae. aegypti and cloned its gene (GenBank accession no. AAL29468) based on partial protein sequences (Han et al., 2002; Han and Li, 2002). Surprisingly, however, its primary sequence shares the highest sequence identity (45–46%) with the mammalian alanine glyoxalate aminotransferases (AGTs) in the GenBank database (GenBank accession nos. CAA53527, AAA31158, NP_057911, BAA02632 for cat, rabbit, mouse, and human, respectively).
3. Functional adaptation and evolution of mosquito AGTs
In mammals, KAT is primarily responsible for the 3-HK to XA pathway. The mosquito KAT (Fang et al., 2002;Han and Li, 2004; Han et al., 2005), sharing high sequence identity (>45%) with mammalian KATs, would logically be considered the probable enzyme responsible for the 3-HK to XA pathway in mosquitoes. In contrast, the protein (GenBank accession no. AAL29468) with high 3-HK transamination activity, shares limited similarity (<10%) with KAT sequences, but has high sequence identity (>45%) with mammalian AGTs. Consequently, the sequence most likely would have been annotated as a mosquito AGT if it had been randomly cloned or determined through a genome project, and its functional assignment as an AGT likely would have been assumed. Apparently, the primary function of this particular mosquito enzyme has shifted or expanded to include the transamination of 3-HK to XA (Fig. 1); accordingly, we named this enzyme Ae. aegypti HKT (Han et al., 2002). The completion of the An. gambiae genome project made it possible to search its database for similar sequences, which led to the discovery of two coding sequences (GenBank accession nos. CAJ14970, and XM_309676) that showed high sequence identity with the Ae. aegypti HKT. BLAST analysis of the Ae. aegypti EST databases also identified a second AGT-like fragment, which subsequently led to the isolation of its full-length cDNA (GenBank accession no.ABA26661). While both An. gambiae and Ae. aegypti possess a second AGT-like sequence, only one AGT is present in other species, including humans. These data raise two interesting questions: why have two AGT-like sequences evolved in mosquitoes and what is the driving force behind the shift in primary function from an AGT to HKT for one of the coding sequences.
3.1. Primary function of AGT
AGT plays an important physiological role in living organisms and has been studied in a variety of animals, plants and fungi. It converts glyoxylate to glycine using pyruvate as the amino group acceptor. The enzyme is present in peroxisomes or mitochondria in different mammalian species. In general, the peroxisomal AGT is responsible for detoxifying glycolate-derived glyoxylate, while the mitochondrial AGT is involved in converting hydroxyproline-derived glyoxylate into glycine (Watts, 1992; Danpure, 1997; Holbrook and Danpure, 2002;Takayama et al., 2003; Birdsey et al., 2004; Birdsey et al., 2005). In plants, it has been shown to be involved in the photorespiratory glyoxylate cycle within peroxisomes (Rehfeld and Tolbert, 1972; Liepman and Olsen, 2001). In yeast, disruption of AGT can lead to glycine auxo-trophy. For example, a yeast AGT deletion strain was unable to grow when glucose was the only carbon source, unless glycine was supplemented (Schlosser et al., 2004). The importance of hepatic AGT in minimizing endogenous oxalate production in some mammals is clearly shown by the autosomal recessive disorder of glyoxylate metabolism known as primary hyperoxaluria type 1. This disorder is a potentially lethal condition in which AGT deficiency leads to excessive oxalate synthesis and excretion and the deposition of insoluble calcium oxalate in the kidney (Danpure et al., 1996; Danpure, 2001). AGT also substantially contributes to the metabolism of serine in humans, dogs, cats, and rabbits, regardless of its localization in mitochondria or in peroxisomes (Rowsell et al., 1979; Beliveau and Freedland, 1982; Xue et al., 1999). Therefore, it is clear that AGT is essential in living organisms from yeast to humans.
3.2. AGT sequences in mosquitoes
By searching an early release of the Ae. aegypti EST sequences (http://www.tigr.org/tdb/e2k1/aabe/) with the Ae. aegypti HKT, we found that Ae. aegypti has an additional AGT-like fragment. This discovery subsequently led to the successful cloning of a new Ae. aegypti AGT (GenBank accession no. ABA26661) that shares 48% identity with the previously reported Ae. aegypti HKT (Han et al., 2002) and ∼45% identity with mammalian AGTs. BLAST analysis of the An. gambiae genomic database revealed that two putative AGT-like sequences with accession numbers of EAA07245 and EAA05410, respectively, also are present in that mosquito species. Recently, the EAA07245 sequence has been identified as the An. gambiae HKT (Rossi et al., 2005). Sequence alignment revealed 73% identity between the two functionally verified proteins, Ae. aegypti HKT (GenBank accession no. AAL29468) and An. gambiae HKT (Gen-Bank accession no. EAA07245), and 80% identity between the second Ae. aegypti (GenBank accession no. ABA26661) and putative An. gambiae AGT (GenBank accession no. EAA05410). Our recent data showed that Ae. aegypti HKT was present only in the larval stages, while its second putative AGT was present during the pupal and adult stages (Han, et al., 2006). BLAST analysis of the genomes of other available model species (including cyanobacteria, archaea, yeast, plants, fruit fly, honeybee, frog, fish, rat, mouse, and human) revealed only one AGT or putative AGT in their databases, which raises essential questions about the physiological roles, functional differentiation, and evolution of mosquito AGTs.
3.3. Functional differences of mosquito AGTs
Based on phylogenetic analysis (Fig. 3), it seems that the functionally verified mosquito HKTs are slightly closer to the mammalian AGTs than the putative Ae. aegypti and An. gambiae AGT sequences (Fig. 3). While our previous study determined that the mosquito HKT is most efficient in catalyzing the transamination of 3-HK to XA, it does have AGT activity (Han et al., 2002; Han and Li, 2002), suggesting that the HKT function might have evolved after the AGT function of the protein or vice versa. Recently, we have expressed the human AGT (GenBank accession no. BAA02632) and initial biochemical characterization of the human enzyme showed that it was highly efficient in mediating the glyoxylate to glycine pathway while also displaying detectable HKT activity (Han et al., 2006). Nonetheless, the ability of human AGT to use 3-HK as a minor substrate suggested the possibility that the mosquito AGT became well-adapted to the 3-HK substrate at its active site during the HKT functional evolution process. In contrast, when the second putative Ae. aegypti AGT sequence (GenBank accession no. ABA26661) was expressed and characterized, the protein displayed high AGT activity, but showed no HKT activity (Han et al., 2006). Interestingly, although the rather extensively characterized mammalian AGTs have been assumed to represent a typical AGT, based on substrate specificity, it is the Ae. aegypti sequence (GenBank accession no. ABA26661) that behaves like a true AGT.
Fig. 3.
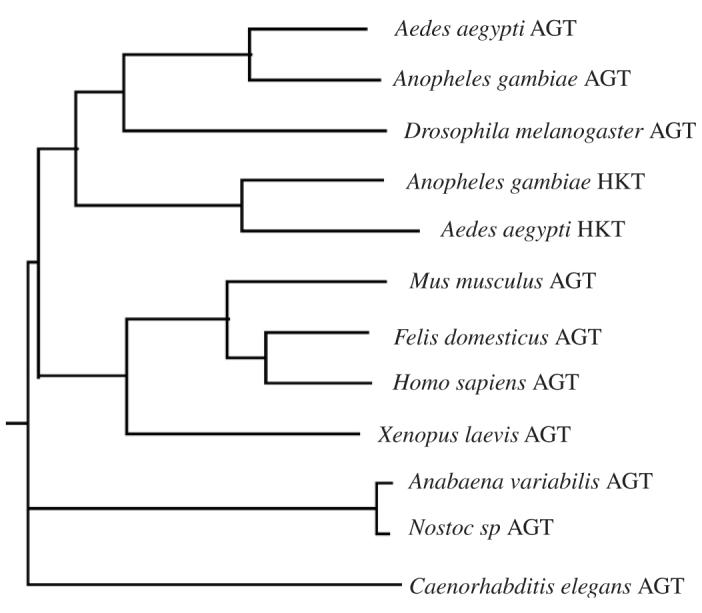
A phylogenetic tree of alanine glyoxalate aminotransferases (AGT) from several representative model species. The phylogenetic tree was generated using CLUSTALW program in biology workbench.
3.4. Possible driving force for mosquito AGT functional adaptation and evolution
Compared with mammalian AGTs, it is clear that one mosquito AGT-like sequence has evolved as HKT and the other has evolved as a true AGT. In mammals, 3-HK can be hydrolyzed by kynureninase (EC 3.7.1.3) to 3-hydroxyanthranilic acid that then can be either completely oxidized to CO2 and H2O through a complicated biochemical pathway or used to synthesize NAD(P)+ (Stone, 1993). Therefore, oxidative stress due to 3-HK accumulation does not seem to be a major problem in mammals. In contrast, mosquitoes do not have kynureninase; therefore, there is no 3-HK hydrolysis pathway available. Consequently, the high conversion efficiency of 3-HK to XA in mosquito larvae is considered to be a mechanism by which mosquitoes detoxify the chemically reactive 3-HK (Han et al., 2002). During mosquito development, larvae have high HKT activity, but this enzyme activity becomes essentially undetectable in pupae and adults. In mosquitoes, as well as other insects, larvae are the active feeding stages. Conceivably, some of the ingested tryptophan will be oxidized via the kynurenine pathway, leading to the production of 3-HK. During the pupal stage, mosquitoes undergo an extensive tissue/body transformation, but do not ingest any food. Accordingly, the tryptophan oxidation pathway likely becomes a minor event, which may explain, in part, the down-regulation of HKT in pupae.
Although 3-HK is toxic in general, it also serves as a precursor for eye pigments in the compound eyes. In mosquitoes, compound eye development occurs primarily during the pupal stages, so some accumulation of 3-HK actually becomes necessary for compound eye pigmentation in the pupal and early adult stages. Indeed, 3-HK becomes a major electrochemically active compound in mosquito pupae. Further analysis indicated that most of the 3-HK was transported to the compound eyes rather than remaining in circulation in the body (Li et al., 1999). These data suggest that the complete down-regulation of mosquito HKT in pupae and adults could be due, in part, to the need for 3-HK for eye pigmentation. The requirements for 3-HK detoxification during the larval stages and the preservation of 3-HK for eye pigmentation during the pupal and early adult stages provide a reasonable explanation for the molecular regulation of the HKT during mosquito development.
Other than mosquitoes, only one AGT or putative AGT sequence is present in other model species (including Drosophila), which logically leads to a question as to why two AGT-like sequences have evolved in mosquitoes. Moreover, based on the protein profile or activity profile in Ae. aegypti, the functionally verified HKT is present in larvae and the second AGT is present in pupae and adults, which is an interesting phenomenon that requires further explanation and justification. Our recent biochemical characterization of the second Ae. aegypti AGT showed that this AGT, unlike mammalian AGTs, functions virtually exclusively on the glyoxylate to glycine pathway (i.e., functions as a true AGT) (Han et al., 2006). It has been shown that AGT is essential in human and yeast (Danpure et al., 1996; Danpure, 2001; Schlosser et al., 2004) and it likely is crucial in insects. In our previous study, we demonstrated that Ae. aegypti HKT, though highly efficient in mediating the 3-HK to XA pathway, also has AGT activity. It is quite possible that the same enzyme is involved in the detoxification of 3-HK and metabolism of glyoxylate during larval development. During the pupal and adult stages, 3-HK needs to be preserved and transported to the compound eyes for eye pigmentation, but the presence of high HKT activity in pupae likely would prevent 3-HK from accumulating. The requirement of 3-HK for eye pigmentation may explain why HKT has to be down-regulated in pupae and adults. On the other hand, if mosquito HKT also plays a role in the glyoxylate to glycine pathway, down-regulating HKT in pupae or adults would affect the glyoxylate metabolism. Western analysis using anti-mosquito AGT antibodies showed that the second Ae. aegypti AGT was present in pupae and adults (Han et al., 2006), but was not detected in larvae. Because the second AGT (GenBank accession no. ABA26661) has no activity for 3-HK, its presence satisfies the need for glyoxylate metabolism without affecting compound eye pigmentation (Han et al., 2006).
The above discussion provides a rather logical explanation as to why mosquitoes have two individual AGTs. Compound eye pigmentation occurs in a number of insect species, including Drosophila. Consequently, one could also ask why Drosophila does not need two AGTs. Drosophila is similar to mosquitoes in that it does not have kynureninase; therefore, accumulation of 3-HK should be a potential problem for Drosophila as well. Analysis of 3-HK and XA in the larval stages revealed that the concentration of 3-HK was about 2–3-fold lower in D. melanogaster larvae than in Ae. aegypti larvae, but the relative concentration of XA was about 20-fold higher in mosquito larvae than in Drosophila larvae (Li and Li, 1997;Li et al., 1999) (Li J., unpublished data). In mosquitoes, larvae are aquatic and microorganisms serve as their primary food resources, so their diets are protein-rich. In contrast, Drosophila larvae get their food from fruit resources, so their diets are rich in carbohydrates and glycolate, the precursor of glyoxylate. It seems clear that mosquito larvae have a significant amount of tryptophan that needs to be dealt with via the kynurenine oxidation pathway. This difference in physiological requirements likely explains why one of the AGT-like sequences in mosquitoes evolved to detoxify 3-HK via transamination.
4. Conclusion
In summary, the study of tryptophan metabolism has revealed that the fates of some tryptophan metabolites are quite different in mosquitoes than those of other species. To prevent 3-HK from accumulating during the larval stages, an AGT-type of enzyme in mosquitoes has expanded its function such that transamination of the chemically reactive 3-HK to the chemically stable XA becomes its primary function or, at least, one of its primary functions. To preserve 3-HK for eye pigmentation and to keep the operation of glyoxylate to glycine during pupal and adult stages, a second AGT, which has no detectable HKT activity and which is highly specific for the transamination of glyoxylate to glycine, has evolved in mosquitoes. These data provide an interesting example of protein functional adaptation and evolution in meeting the physiological requirements of mosquitoes.
Acknowledgments
This work was supported by a National Institutes of Health Grant (AI 44399).
References
- Akaboshi E. Kynurenine hydroxylase in Musca domestica L. Comparative Biochemistry and Physiology B. 1979;62:549–555. doi: 10.1016/0305-0491(79)90132-9. [DOI] [PubMed] [Google Scholar]
- Arndt R, Junge W, Michelssen K, Krisch K. Isolation and molecular properties of formamidase from rat liver cytoplasm. HoppeSeyler’s Zeitschrift für Physiologische Chemie. 1973;354:1583–1590. doi: 10.1515/bchm2.1973.354.2.1583. [DOI] [PubMed] [Google Scholar]
- Atkinson PW, Pinkerton AC, O’Brochta DA. Genetic transformation systems in insects. Annual Review of Entomology. 2001;46:317–346. doi: 10.1146/annurev.ento.46.1.317. [DOI] [PubMed] [Google Scholar]
- Bailey CG, Wagner C. Kynurenine formamidase. Purification and characterization of the adult chicken liver enzyme and immuno-chemical analyses of the enzyme of developing chicks. Journal of Biological Chemistry. 1974;249:4439–4444. [PubMed] [Google Scholar]
- Baillie DL, Chovnick A. Studies on the genetic control of tryptophan pyrrolase in Drosophila melanogaster. Molecular and General Genetics. 1971;112:341–353. doi: 10.1007/BF00334435. [DOI] [PubMed] [Google Scholar]
- Beard CB, Benedict MQ, Primus JP, Finnerty V, Collins FH. Eye pigments in wild-type and eye-color mutant strains of the African malaria vector Anopheles gambiae. Journal of Heredity. 1995;86:375–380. doi: 10.1093/oxfordjournals.jhered.a111606. [DOI] [PubMed] [Google Scholar]
- Beliveau GP, Freedland RA. Metabolism of serine, glycine and threonine in isolated cat hepatocytes Felis domestica. Comparative Biochemistry and Physiology B. 1982;71:13–18. doi: 10.1016/0305-0491(82)90168-7. [DOI] [PubMed] [Google Scholar]
- Bhalla SC. Genetic aspects of pteridines in mosquitoes. Genetics. 1968;58:249–258. doi: 10.1093/genetics/58.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billker O, Lindo V, Panico M, Etienne AE, Paxton T, Dell A, Rogers M, Sinden RE, Morris HR. Identification of xanthurenic acid as the putative inducer of malaria development in the mosquito. Nature. 1998;392:289–292. doi: 10.1038/32667. [DOI] [PubMed] [Google Scholar]
- Birdsey GM, Lewin J, Cunningham AA, Bruford MW, Danpure CJ. Differential enzyme targeting as an evolutionary adaptation to herbivory in carnivora. Molecular Biology and Evolution. 2004;21:632–646. doi: 10.1093/molbev/msh054. [DOI] [PubMed] [Google Scholar]
- Birdsey GM, Lewin J, Holbrook JD, Simpson VR, Cunningham AA, Danpure CJ. A comparative analysis of the evolutionary relationship between diet and enzyme targeting in bats, marsupials and other mammals. Proceedings of the Royal Society B: Biological Sciences. 2005;272:833–840. doi: 10.1098/rspb.2004.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady FO, Forman HJ, Feigelson P. The role of superoxide and hydroperoxide in the reductive activation of tryptophan-2,3-dioxygenase. Journal of Biological Chemistry. 1971;246:7119–7124. [PubMed] [Google Scholar]
- Breton J, Avanzi N, Magagnin S, Covini N, Magistrelli G, Cozzi L, Isacchi A. Functional characterization and mechanism of action of recombinant human kynurenine 3-hydroxylase. European Journal of Biochemistry. 2000;267:1092–1099. doi: 10.1046/j.1432-1327.2000.01104.x. [DOI] [PubMed] [Google Scholar]
- Brown D, Hitchcock MJ, Katz E. Purification and characterization of kynurenine formamidase activities from Streptomyces parvulus. Canadian Journal of Microbiology. 1986;32:465–472. doi: 10.1139/m86-086. [DOI] [PubMed] [Google Scholar]
- Burnet B, Sang JH. Physiological genetics of melanotic tumors in Drosophila melanogaster, V: amino acid metabolism and tumor formation in the tu bw; st su-tu strain. Genetics. 1968;59:211–235. doi: 10.1093/genetics/59.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerstiaens A, Huybrechts J, Kotanen S, Lebeau I, Meylaers K, De Loof A, Schoofs L. Neurotoxic and neurobehavioral effects of kynurenines in adult insects. Biochemical and Biophysical Research Communication. 2003;312:1171–1177. doi: 10.1016/j.bbrc.2003.11.051. [DOI] [PubMed] [Google Scholar]
- Coates CJ, Jasinskiene N, Miyashiro L, James AA. Mariner transposition and transformation of the yellow fever mosquito, Aedes aegypti. Proceedings of the National Academy of Sciences, USA. 1998;95:3748–3751. doi: 10.1073/pnas.95.7.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornel AJ, Benedict MQ, Rafferty CS, Howells AJ, Collins FH. Transient expression of the Drosophila melanogaster cinnabar gene rescues eye color in the white eye (WE) strain of Aedes aegypti. Insect Biochemistry Molecular Biology. 1997;27:993–997. doi: 10.1016/s0965-1748(97)00084-2. [DOI] [PubMed] [Google Scholar]
- Danpure CJ. Variable peroxisomal and mitochondrial targeting of alanine: glyoxylate aminotransferase in mammalian evolution and disease. Bioessays. 1997;19:317–326. doi: 10.1002/bies.950190409. [DOI] [PubMed] [Google Scholar]
- Danpure CJ. Primary hyperoxaluria. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler KW, Vogelstein B, editors. The Molecular and Metabolic Bases of Inherited Disease. McGraw-Hill; New York: 2001. pp. 3323–3367. [Google Scholar]
- Danpure CJ, Jennings PR, Leiper JM, Lumb MJ, Oatey PB. Targeting of alanine: glyoxylate aminotransferase in normal individuals and its mistargeting in patients with primary hyper-oxaluria type 1. Annals of the New York Academy of Sciences. 1996;804:477–490. doi: 10.1111/j.1749-6632.1996.tb18638.x. [DOI] [PubMed] [Google Scholar]
- Dick R, Murray BP, Reid MJ, Correia MA. Structure–function relationships of rat hepatic tryptophan 2,3-dioxygenase: identification of the putative heme-ligating histidine residues. Archives of Biochemistry and Biophysics. 2001;392:71–78. doi: 10.1006/abbi.2001.2420. [DOI] [PubMed] [Google Scholar]
- Entsch B, Ballou DP, Husain M, Massey V. Catalytic mechanism of p-hydroxybenzoate hydroxylase with p-mercaptobenzoate as substrate. Journal of Biological Chemistry. 1976a;251:7367–7369. [PubMed] [Google Scholar]
- Entsch B, Ballou DP, Massey V. Flavin-oxygen derivatives involved in hydroxylation by p-hydroxybenzoate hydroxylase. Journal of Biological Chemistry. 1976b;251:2550–2563. [PubMed] [Google Scholar]
- Eppink MH, Schreuder HA, Van Berkel WJ. Identification of a novel conserved sequence motif in flavoprotein hydroxylases with a putative dual function in FAD/NAD(P)H binding. Protein Science. 1997;6:2454–2458. doi: 10.1002/pro.5560061119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Han Q, Li J. Isolation, characterization, and functional expression of kynurenine aminotransferase cDNA from the yellow fever mosquito, Aedes aegypti. Insect Biochemistry and Molecular Biology. 2002;32:943–950. doi: 10.1016/s0965-1748(02)00032-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia GE, Wirtz RA, Barr JR, Woolfitt A, Rosenberg R. Xanthurenic acid induces gametogenesis in Plasmodium, the malaria parasite. Journal of Biological Chemistry. 1998;273:12003–12005. doi: 10.1074/jbc.273.20.12003. [DOI] [PubMed] [Google Scholar]
- Guidetti P, Okuno E, Schwarcz R. Characterization of rat brain kynurenine aminotransferases I and II. Journal of Neuroscience Research. 1997;50:457–465. doi: 10.1002/(SICI)1097-4547(19971101)50:3<457::AID-JNR12>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Han Q, Li J. Comparative characterization of Aedes 3-hydroxykynurenine transaminase/alanine glyoxylate transaminase and Drosophila serine pyruvate aminotransferase. FEBS Letters. 2002;527:199–204. doi: 10.1016/s0014-5793(02)03229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Q, Li J. Cysteine and keto acids modulate mosquito kynurenine aminotransferase catalyzed kynurenic acid production. FEBS Letters. 2004;577:381–385. doi: 10.1016/j.febslet.2004.09.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Q, Fang J, Li J. 3-Hydroxykynurenine transaminase identity with alanine glyoxylate transaminase. A probable detoxification protein in Aedes aegypti. Journal of Biological Chemistry. 2002;277:15781–15787. doi: 10.1074/jbc.M201202200. [DOI] [PubMed] [Google Scholar]
- Han Q, Calvo E, Marinotti O, Fang J, Rizzi M, James AA, Li J. Analysis of the wild-type and mutant genes encoding the enzyme kynurenine monooxygenase of the yellow fever mosquito, Aedes aegypti. Insect Molecular Biology. 2003;12:483–490. doi: 10.1046/j.1365-2583.2003.00433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Q, Li J, Li J. pH dependence, substrate specificity and inhibition of human kynurenine aminotransferase I. European Journal of Biochemistry. 2004;271:4804–4814. doi: 10.1111/j.1432-1033.2004.04446.x. [DOI] [PubMed] [Google Scholar]
- Han Q, Gao YG, Robinson H, Ding H, Wilson S, Li J. Crystal structures of Aedes aegypti kynurenine aminotransferase. FEBS Journal. 2005;272:2198–2206. doi: 10.1111/j.1742-4658.2005.04643.x. [DOI] [PubMed] [Google Scholar]
- Han Q, Kim SR, Ding H, Li J. Evolution of two alanine glyoxylate aminotransferases in mosquito. Biochemical Journal. 2006;397:473–481. doi: 10.1042/BJ20060469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayaishi O, Nozaki M. Nature and mechanisms of oxygenases. Science. 1969;164:389–396. doi: 10.1126/science.164.3878.389. [DOI] [PubMed] [Google Scholar]
- Hirai M, Kiuchi M, Wang J, Ishii A, Matsuoka H. cDNA cloning, functional expression and characterization of kynurenine 3-hydroxylase of Anopheles stephensi (Diptera: Culicidae) Insect Molecular Biology. 2002;11:497–504. doi: 10.1046/j.1365-2583.2002.00358.x. [DOI] [PubMed] [Google Scholar]
- Hitchcock MJ, Katz E. Purification and characterization of tryptophan dioxygenase from Streptomyces parvulus. Archives of Biochemistry and Biophysics. 1988;261:148–160. doi: 10.1016/0003-9861(88)90113-0. [DOI] [PubMed] [Google Scholar]
- Holbrook JD, Danpure CJ. Molecular basis for the dual mitochondrial and cytosolic localization of alanine:glyoxylate amino-transferase in amphibian liver cells. Journal of Biological Chemistry. 2002;277:2336–2344. doi: 10.1074/jbc.M107047200. [DOI] [PubMed] [Google Scholar]
- Howells AJ. Isolation and biochemical analysis of a temperature-sensitive scarlet eye color mutant of Drosophila melanogaster. Biochemical Genetics. 1979;17:149–158. doi: 10.1007/BF00484480. [DOI] [PubMed] [Google Scholar]
- Howells AJ, Summers KM, Ryall RL. Developmental patterns of 3-hydroxykynurenine accumulation in white and various other eye color mutants of Drosophila melanogaster. Biochemical Genetics. 1977;15:1049–1059. doi: 10.1007/BF00484496. [DOI] [PubMed] [Google Scholar]
- Ishimura Y, Hayaishi O. Noninvolvement of copper in the Ltryptophan 2,3-digoxygenase reaction. Journal of Biological Chemistry. 1973;248:8610–8612. [PubMed] [Google Scholar]
- Jacobson KB. Role of an isoacceptor transfer ribonucleic acid as an enzyme inhibitor: effect on tryptophan pyrrolase of Drosophila. Nature New Biology. 1971;231:17–19. [PubMed] [Google Scholar]
- Jasinskiene N, Coates CJ, Benedict MQ, Cornel AJ, Rafferty CS, James AA, Collins FH. Stable transformation of the yellow fever mosquito, Aedes aegypti, with the Hermes element from the housefly. Proceedings of the National Academy of Sciences, USA. 1998;95:3743–3747. doi: 10.1073/pnas.95.7.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman S. Studies on tryptophan pyrrolase in Drosophila melanogaster. Genetics. 1962;47:807–817. doi: 10.1093/genetics/47.7.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Hayashi K, Sono M. Effects of tryptophan and pH on the kinetics of superoxide radical binding to indoleamine 2,3-dioxygenase studied by pulse radiolysis. Journal of Biological Chemistry. 1989;264:15280–15283. [PubMed] [Google Scholar]
- Koike K, Poillon WN, Feigelson P. Influence of allosteric effector substances on the structure and catalytic acitivity of tryptophan oxygenase. Journal of Biological Chemistry. 1969;244:3457–3462. [PubMed] [Google Scholar]
- Kurnasov O, Jablonski L, Polanuyer B, Dorrestein P, Begley T, Osterman A. Aerobic tryptophan degradation pathway in bacteria: novel kynurenine formamidase. FEMS Microbiology Letters. 2003;227:219–227. doi: 10.1016/S0378-1097(03)00684-0. [DOI] [PubMed] [Google Scholar]
- Leeds JM, Brown PJ, McGeehan GM, Brown FK, Wiseman JS. Isotope effects and alternative substrate reactivities for tryptophan 2,3-dioxygenase. Journal of Biological Chemistry. 1993;268:17781–17786. [PubMed] [Google Scholar]
- Li J, Li G. Transamination of 3-hydroxykynurenine to produce xanthurenic acid: a major branch pathway of tryptophan metabolism in the mosquito, Aedes aegypti, during larval development. Insect Biochemistry and Molecular Biology. 1997;27:859–867. doi: 10.1016/s0965-1748(97)00068-4. [DOI] [PubMed] [Google Scholar]
- Li J, Li G. Identification of 3-hydroxykynurenine and xanthurenic acid and quantitation of 3-hydroxykynurenine transaminase activity using HPLC with electrochemical detection. Journal of Liquid Chromatography & Related Technologies. 1998;21:1511–1525. [Google Scholar]
- Li J, Beerntsen BT, James AA. Oxidation of 3-hydroxykynurenine to produce xanthommatin for eye pigmentation: a major branch pathway of tryptophan catabolism during pupal development in the yellow fever mosquito, Aedes aegypti. Insect Biochemistry and Molecular Biology. 1999;29:329–338. doi: 10.1016/s0965-1748(99)00007-7. [DOI] [PubMed] [Google Scholar]
- Liepman AH, Olsen LJ. Peroxisomal alanine: glyoxylate aminotransferase (AGT1) is a photorespiratory enzyme with multiple substrates in Arabidopsis thaliana. Plant Journal. 2001;25:487–498. doi: 10.1046/j.1365-313x.2001.00961.x. [DOI] [PubMed] [Google Scholar]
- Lorenzen MD, Brown SJ, Denell RE, Beeman RW. Cloning and characterization of the Tribolium castaneum eye-color genes encoding tryptophan oxygenase and kynurenine 3-monooxygenase. Genetics. 2002;160:225–234. doi: 10.1093/genetics/160.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzen B. The tryptophan-ommochrome pathway in insects. Advances in Insect Physiology. 1974;10:117–246. [Google Scholar]
- Marzluf GA. Tryptophan pyrrolase of Drosophila: partial purification and properties. Zeitschrift für Vererbungslehre. 1965;97:10–17. doi: 10.1007/BF00898568. [DOI] [PubMed] [Google Scholar]
- Mehler AH, Knox WE. The conversion of tryptophan to kynurenine in liver, II: the enzymatic hydrolysis of formylkynurenine. Journal of Biological Chemistry. 1950;187:431–438. [PubMed] [Google Scholar]
- Mischke D, Kloetzel P, Schwochau M. Tryptophan pyrrolase activity regulation in Drosophila: role of an isoacceptor tRNA unsettled. Nature. 1975;255:79–80. doi: 10.1038/255079a0. [DOI] [PubMed] [Google Scholar]
- Moore GP, Sullivan DT. The characterization of multiple forms of kynurenine formidase in Drosophila melanogaster. Biochimica et Biophysica Acta. 1975;397:468–477. doi: 10.1016/0005-2744(75)90137-0. [DOI] [PubMed] [Google Scholar]
- Moore GP, Sullivan DT. Biochemical and genetic characterization of kynurenine formamidase from Drosophila melanogaster. Biochemical Genetics. 1978;16:619–634. doi: 10.1007/BF00484718. [DOI] [PubMed] [Google Scholar]
- Moroni F. Tryptophan metabolism and brain function: focus on kynurenine and other indole metabolites. European Journal of Pharmacology. 1999;375:87–100. doi: 10.1016/s0014-2999(99)00196-x. [DOI] [PubMed] [Google Scholar]
- Moroni F, Cozzi A, Carpendo R, Cipriani G, Veneroni O, Izzo E. Kynurenine 3-mono-oxygenase inhibitors reduce glutamate concentration in the extracellular spaces of the basal ganglia but not in those of the cortex or hippocampus. Neuropharmacology. 2005;48:788–795. doi: 10.1016/j.neuropharm.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Mukabayire O, Cornel AJ, Dotson EM, Collins FH, Besansky NJ. The Tryptophan oxygenase gene of Anopheles gambiae. Insect Biochemistry and Molecular Biology. 1996;26:525–528. doi: 10.1016/s0965-1748(96)00026-4. [DOI] [PubMed] [Google Scholar]
- Okuda S, Nishiyama N, Saito H, Katsuki H. Hydrogen peroxide-mediated neuronal cell death induced by an endogenous neurotoxin, 3-hydroxykynurenine. Proceedings of the National Academy of Sciences, USA. 1996;93:12553–12558. doi: 10.1073/pnas.93.22.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda S, Nishiyama N, Saito H, Katsuki H. 3-Hydroxykynurenine, an endogenous oxidative stress generator, causes neuronal cell death with apoptotic features and region selectivity. Journal of Neurochemistry. 1998;70:299–307. doi: 10.1046/j.1471-4159.1998.70010299.x. [DOI] [PubMed] [Google Scholar]
- Okuno E, Nakamura M, Schwarcz R. Two kynurenine aminotransferases in human brain. Brain Research. 1991;542:307–312. doi: 10.1016/0006-8993(91)91583-m. [DOI] [PubMed] [Google Scholar]
- Pabarcus MK, Casida JE. Kynurenine formamidase: determination of primary structure and modeling-based prediction of tertiary structure and catalytic triad. Biochimica et Biophysica Acta. 2002;1596:201–211. doi: 10.1016/s0167-4838(02)00232-7. [DOI] [PubMed] [Google Scholar]
- Paton DR, Sullivan DT. Mutagenesis at the cinnabar locus in Drosophila melanogaster. Biochemical Genetics. 1978;16:855–865. doi: 10.1007/BF00483738. [DOI] [PubMed] [Google Scholar]
- Pellicciari R, Amori L, Costantino G, Giordani A, Macchiarulo A, Mattoli L, Pevarello P, Speciale C, Varasi M. Modulation of the kynurine pathway of tryptophan metabolism in search for neuroprotective agents: focus on kynurenine-3-hydroxylase. Advances in Experimental Medicine and Biology. 2003;527:621–628. doi: 10.1007/978-1-4615-0135-0_71. [DOI] [PubMed] [Google Scholar]
- Phillips JP, Simmons JR, Bowman JT. Re-evaluation of a system for the in vitro synthesis of tryptophan pyrrolase. Biochemical and Biophysical Research Communication. 1967;29:253–257. doi: 10.1016/0006-291x(67)90444-5. [DOI] [PubMed] [Google Scholar]
- Quan GX, Kim I, Komoto N, Sezutsu H, Ote M, Shimada T, Kanda T, Mita K, Kobayashi M, Tamura T. Characterization of the kynurenine 3-monooxygenase gene corresponding to the white egg 1 mutant in the silkworm Bombyx mori. Molecular Genetics and Genomics. 2002;267:1–9. doi: 10.1007/s00438-001-0629-2. [DOI] [PubMed] [Google Scholar]
- Rasgon JL, Scott TW. Crimson: a novel sex-linked eye color mutant of Culex pipiens L. (Diptera: Culicidae) Journal of Medical Entomology. 2004;41:385–391. doi: 10.1603/0022-2585-41.3.385. [DOI] [PubMed] [Google Scholar]
- Rehfeld DW, Tolbert NE. Aminotransferases in peroxisomes from spinach leaves. Journal of Biological Chemistry. 1972;247:4803–4811. [PubMed] [Google Scholar]
- Rossi F, Lombardo F, Paglino A, Cassani C, Miglio G, Arca B, Rizzi M. Identification and biochemical characterization of the Anopheles gambiae 3-hydroxykynurenine transaminase. FEBS Journal. 2005;272:5653–5662. doi: 10.1111/j.1742-4658.2005.04961.x. [DOI] [PubMed] [Google Scholar]
- Rossi F, Garavaglia S, Giovenzana GB, Arca B, Li J, Rizzi M. Crystal structure of the Anopheles gambiae 3-hydroxykynurenine transaminase. Proceedings of the National Academy of Sciences, USA. 2006;103:5711–5716. doi: 10.1073/pnas.0510233103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowsell EV, Carnie JA, Wahbi SD, Al-Tai AH, Rowsell KV. L-serine dehydratase and L-serine-pyruvate aminotransferase activities in different animal species. Comparative Biochemistry and Physiology B. 1979;63:543–555. doi: 10.1016/0305-0491(79)90061-0. [DOI] [PubMed] [Google Scholar]
- Samadi P, Gregoire L, Rassoulpour A, Guidetti P, Izzo E, Schwarcz R, Bedard PJ. Effect of kynurenine 3-hydroxylase inhibition on the dyskinetic and antiparkinsonian responses to levodopa in Parkinsonian monkeys. Movement Disorders. 2005;20:792–802. doi: 10.1002/mds.20596. [DOI] [PubMed] [Google Scholar]
- Schlosser T, Gatgens C, Weber U, Stahmann KP. Alanine: glyoxylate aminotransferase of Saccharomyces cerevisiae-encoding gene AGX1 and metabolic significance. Yeast. 2004;21:63–73. doi: 10.1002/yea.1058. [DOI] [PubMed] [Google Scholar]
- Schutz G, Chow E, Feigelson P. Regulatory properties of hepatic tryptophan oxygenase. Journal of Biological Chemistry. 1972;247:5333–5337. [PubMed] [Google Scholar]
- Schwarcz R. Metabolism and function of brain kynurenines. Biochemical Society Transactions. 1993;21:77–82. doi: 10.1042/bst0210077. [DOI] [PubMed] [Google Scholar]
- Schwarcz R. The kynurenine pathway of tryptophan degradation as a drug target. Current Opinion in Pharmacology. 2004;4:12–17. doi: 10.1016/j.coph.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Searles LL, Voelker RA. Molecular characterization of the Drosophila vermilion locus and its suppressible alleles. Proceedings of the National Academy of Sciences, USA. 1986;83:404–408. doi: 10.1073/pnas.83.2.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searles LL, Ruth RS, Pret AM, Fridell RA, Ali AJ. Structure and transcription of the Drosophila melanogaster vermilion gene and several mutant alleles. Molecular Cell Biology. 1990;10:1423–1431. doi: 10.1128/mcb.10.4.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethuraman N, O’Brochta DA. The Drosophila melanogaster cinnabar gene is a cell autonomous genetic marker in Aedes aegypti (Diptera: Culicidae) Journal of Medical Entomology. 2005;42:716–718. doi: 10.1093/jmedent/42.4.716. [DOI] [PubMed] [Google Scholar]
- Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone TW. Neuropharmacology of quinolinic and kynurenic acids. Pharmacological Reviews. 1993;45:309–379. [PubMed] [Google Scholar]
- Stone TW. Development and therapeutic potential of kynurenic acid and kynurenine derivatives for neuroprotection. Trends in Pharmacological Sciences. 2000;21:149–154. doi: 10.1016/s0165-6147(00)01451-6. [DOI] [PubMed] [Google Scholar]
- Stone TW. Kynurenic acid antagonists and kynurenine pathway inhibitors. Expert Opinion on Investigational Drugs. 2001a;10:633–645. doi: 10.1517/13543784.10.4.633. [DOI] [PubMed] [Google Scholar]
- Stone TW. Endogenous neurotoxins from tryptophan. Toxicon. 2001b;39:61–73. doi: 10.1016/s0041-0101(00)00156-2. [DOI] [PubMed] [Google Scholar]
- Stone TW. Kynurenines in the CNS: from endogenous obscurity to therapeutic importance. Progress in Neurobiology. 2001c;64:185–218. doi: 10.1016/s0301-0082(00)00032-0. [DOI] [PubMed] [Google Scholar]
- Stone TW, Darlington LG. Endogenous kynurenines as targets for drug discovery and development. Nature Reviews Drug Discovery. 2002;1:609–620. doi: 10.1038/nrd870. [DOI] [PubMed] [Google Scholar]
- Stone TW, MacGregor DG, Smith RA, Jones P, Behan WM, Graham DI. Basic mechanisms of kynurenine actions in the central nervous system. Advances in Experimental Medicine and Biology. 1996;398:195–201. doi: 10.1007/978-1-4613-0381-7_32. [DOI] [PubMed] [Google Scholar]
- Sullivan DT, Grillo SL, Kitos RJ. Subcellular localization of the first three enzymes of the ommochrome synthetic pathway in Drosophila melanogaster. Journal of Experimental Zoology. 1974;188:225–233. doi: 10.1002/jez.1401880210. [DOI] [PubMed] [Google Scholar]
- Summers KM, Howells AJ. Xanthommatin biosynthesis in wild-type and mutant strains of the Australian sheep blowfly Lucilia cuprina. Biochemical Genetics. 1978;16:1153–1163. doi: 10.1007/BF00484536. [DOI] [PubMed] [Google Scholar]
- Takayama T, Fujita K, Suzuki K, Sakaguchi M, Fujie M, Nagai E, Watanabe S, Ichiyama A, Ogawa Y. Control of oxalate formation from L-hydroxyproline in liver mitochondria. Journal of American Society of Nephrology. 2003;14:939–946. doi: 10.1097/01.asn.0000059310.67812.4f. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Knox WE. The nature and mechanism of the tryptophan pyrrolase (peroxidase-oxidase) reaction of Pseudomonas and of rat liver. Journal of Biological Chemistry. 1959;234:1162–1170. [PubMed] [Google Scholar]
- Tartof KD. Interacting gene systems, I: the regulation of tryptophan pyrrolase by the vermilion-suppressor of vermilion system in Drosophila. Genetics. 1969;62:781–795. [PMC free article] [PubMed] [Google Scholar]
- Tobler JE. Dosage compensation and ontogenic expression of suppressed and transformed Vermilion flies in Drosophila. Biochemical Genetics. 1975;13:29–43. doi: 10.1007/BF00486004. [DOI] [PubMed] [Google Scholar]
- Tobler J, Bowman JT, Simmons JR. Gene modulation in Drosophila: dosage compensation and relocated v+genes. Biochemical Genetics. 1971;5:111–117. doi: 10.1007/BF00485639. [DOI] [PubMed] [Google Scholar]
- Walker AR, Howells AJ, Tearle RG. Cloning and characterization of the vermilion gene of Drosophila melanogaster. Molecular and General Genetics. 1986;202:102–107. [Google Scholar]
- Warren WD, Palmer S, Howells AJ. Molecular characterization of the cinnabar region of Drosophila melanogaster: identification of the cinnabar transcription unit. Genetica. 1996;98:249–262. doi: 10.1007/BF00057589. [DOI] [PubMed] [Google Scholar]
- Watts RW. Alanine glyoxylate aminotransferase deficiency: biochemical and molecular genetic lessons from the study of a human disease. Advances in Enzyme Regulation. 1992;32:309–327. doi: 10.1016/0065-2571(92)90024-t. [DOI] [PubMed] [Google Scholar]
- Wei H, Leeds P, Chen RW, Wei W, Leng Y, Bredesen DE, Chuang DM. Neuronal apoptosis induced by pharmacological concentrations of 3-hydroxykynurenine: characterization and protection by dantrolene and Bcl-2 overexpression. Journal of Neurochemistry. 2000;75:81–90. doi: 10.1046/j.1471-4159.2000.0750081.x. [DOI] [PubMed] [Google Scholar]
- Xue HH, Sakaguchi T, Fujie M, Ogawa H, Ichiyama A. Flux of the L-serine metabolism in rabbit, human, and dog livers: substantial contributions of both mitochondrial and peroxisomal serine:pyruvate/alanine:glyoxylate aminotransferase. Journal of Biological Chemistry. 1999;274:16028–16033. doi: 10.1074/jbc.274.23.16028. [DOI] [PubMed] [Google Scholar]