Summary
With increasing age, the ability to produce protective antibodies in response to immunization declines, resulting in reduced efficacy of vaccination. We have examined how reductions in CD4+ T-cell function contribute to reduced humoral responses, using a model that allows us to compare identical numbers of antigen-specific naive T cells from young and aged T-cell receptor transgenic mice. Naive cells from aged mice exhibit reduced responses, both in vitro and in vivo. In vitro, responses of aged T cells can be enhanced by addition of interleukin (IL)-2. In vivo, using an adoptive transfer model with young hosts, naive cells from aged mice exhibit significant reductions in cognate helper function, leading to reduced B-cell expansion and differentiation. These age-related defects could be overcome by prior in vitro T helper 2 effector generation with aged T cells. This improvement in cognate function of the aged effectors may be related to the enhancement of CD154 expression, which occurs on aged T cells in the presence of exogenous IL-2. We also found no difference in B-cell expansion and differentiation when young cells were transferred to young or aged hosts. Our results indicate that age-related reductions in humoral responses are mainly due to defects in the cognate helper function of naive CD4+ T cells from aged individuals.
Introduction
Increased morbidity and mortality seen in elderly populations following infection are thought to be the result of age-associated changes in the immune system. One of the most prominent and dramatic changes is the reduced efficacy of vaccinations in the elderly. In humans, studies have focused on antibody production in response to vaccination and have shown that the efficacy of vaccinations for Streptococcus pneumoniae, influenza, hepatitis, and tetanus are all greatly decreased with age (1–4). These studies also found that the amount and the function of antibodies produced in the elderly are decreased. Moreover, age-related defects in the immune system have been shown to lead to reduced germinal center (GC) formation, decreased levels of somatic mutation, and the production of antibodies that are less protective (5–9). This is especially problematic, because the elderly are oftentimes targeted for vaccinations. Studies in mice have also correlated reduced GC formation with declining humoral responses. Both the number as well as the volume of GCs have been shown to decline slowly with increasing age, resulting in reduced affinity maturation of antibodies (9). This phenomenon is also likely to account for the observed reductions in the establishment of long-lived antibody-producing cells in the bone marrow (10) and the generation of memory B-cell populations (11), both of which are necessary for adequate continuing protection from infection.
Because the cognate helper activity of CD4+ T cells is critical for GC formation as well as proper antibody production and function, it has been proposed that declines in CD4+ T-cell function are somehow involved in these observed age-related changes (9). GC formation requires antigen-specific CD4+ T cells, antigen-specific B cells, and follicular dendritic cells (FDCs). GCs consist of rapidly proliferating B cells and are the site of antibody isotype switching, antibody V gene hypermutation, and memory B-cell development (11). While GC formation has been extensively studied, it is not yet fully understood (11–13). At the initiation of an antigen-specific response, CD4+ T cells respond to antigen presented to them in the T-cell zone and, at the same time, responding B cells undergo initial cognate interactions with these T cells. A select proportion of these responding B cells are then signaled to migrate back to the B-cell follicle, where they interact with FDCs presenting the specific antigen. The mechanisms governing which B cells are selected to do this are still unclear. In the B-cell follicle, the responding B cells interact with FDCs, undergo many rounds of proliferation, and then differentiate to a prototypical GC phenotype. During this process, most of the responding B cells undergo apoptosis, and only those expressing appropriate high-affinity receptors are selected to continue on in the response (14). Following this initial phase, antibody V gene hypermutation occurs (15, 16), resulting in the generation of a diverse antibody repertoire, which is necessary for optimal responses to invading pathogens.
While it is obvious that age-related defects can influence one or several of the steps in the formation of the GC, published studies have suggested that a main defect lies within the CD4+ T-helper population. Adoptive transfer studies of bulk populations of aged CD4+ T cells and young B cells into severe combined immunodeficiency hosts have shown that the aged CD4+ T cells do not provide adequate help (17, 18). This lack results in reduced clonal competition between responding B cells, leading to the production of lower affinity antibodies. Additionally, significant changes in antibody V gene usage have been observed in aged mice, which also contribute to repertoire changes (17). Based on these results, our work has focused on how age influences the cognate helper function of naive antigen-specific CD4+ T cells. Importantly, as discussed below, our model is novel as it allows us to examine the function of equal numbers of antigen-specific CD4+ T cells from young and aged mice, thus eliminating other variables present in the previous studies.
Effect of age on the in vitro function of naive CD4+ T cells
Aging has a dramatic impact on the in vitro function of T cells. Some of the responses that have been examined were found to decline with age include proliferation in response to T-cell receptor (TCR) stimulation and T-cell mitogens, interleukin (IL)-2 production, and cytotoxic T-lymphocyte generation (19). These defects are likely due to reductions in TCR signaling pathways in aged T cells. Reductions have been observed in a multitude of signaling pathways including calcium mobilization and tyrosine phosphorylation (20–22) as well as nuclear factor κB (NFκB) and nuclear factor of activated T cells translocation (23, 24). Another of the most prominent changes is that as an individual age, the percentage of naive T cells in the periphery declines and the percentage of memory T cells increases (25–27). This outcome is due to both a dramatic decline in new T-cell production with age and a lifetime of exposure to antigens. One of the consequences is that the repertoire of naive T cells available to respond to newly encountered antigen declines with increasing age. This point is critical, as differences in proportions of naive and memory cells also complicate the interpretation of experimental results, and is especially evident, as memory cells exhibit differences in response to TCR signaling as well as differences in cytokine production, when compared with naive T cells (28). Thus, it is clear that purified populations of naive CD4+ T cells from young and aged individuals need to be used for functional studies both in vitro and in vivo.
Those naive cells that do remain in aged animals exhibit reduced in vitro function in response to stimulus. Elegant studies from Garcia and Miller (23, 29) have shown that naive CD4+ T cells from aged TCR transgenic (TCR Tg) mice do not form immunological synapses with antigen-presenting cells (APCs) as efficiently as cells from young mice. Their data also demonstrate that aging leads to decreased translocation of TCR-associated proteins to the immunological synapse. This reduction in recruitment of signaling molecules in aged naive CD4+ T cells is reduced by approximately 50% compared with young. Furthermore, these authors have also have shown that naive CD4+ T cells from aged mice have significant changes in cytoskeletal rearrangement and cell-surface glycosylation that also contribute to reduced function (30, 31). These age-related changes, which seem to originate at the cell membrane, result in the quality of the initial TCR signal being reduced in the naive CD4+ T cells from aged animals, which then results in many downstream reductions in the response.
Our hypothesis is that defects in function are due to the fact that naive CD4+ T cells in aged mice are chronologically older than those in young mice, and we have proposed a model to explain why the function of naive CD4+ T cells declines with increasing age (Fig. 1). Young mice exhibit robust production of new T cells, and as the size of the peripheral T-cell pool remains constant, there must be a great deal of turnover in the periphery. New cells come in and older cells are cleared from the circulation. As mice age, thymic involution occurs, which results in a dramatic decline in the production of new T cells (32, 33). Consequently, the amount of time each cell spends in the periphery must be increased, as the number of peripheral T cells remains constant. Thus, the naive T cells in the circulation of older animals are mostly older than those in younger animals. We hypothesize that the longer these naive T cells spend in the periphery, the greater the decline in function. This idea would account for the age-related changes that have been observed in immunological synapse formation in naive cells (23, 29). These defects may be due to a number of factors and probably occur because of age-related changes in the cell membrane. For example, it has been reported that exposure of naive T cells to oxidative damage can affect lipid membranes, and it has been shown to cause decreases in IL-2 production by naive T cells (34). Thus, it is quite probable that naive CD4+ T-cell function declines as a consequence of the increasing age of the T cells themselves.
Fig. 1. Model for how defects in naive CD4+ T-cell function occur with increasing age.
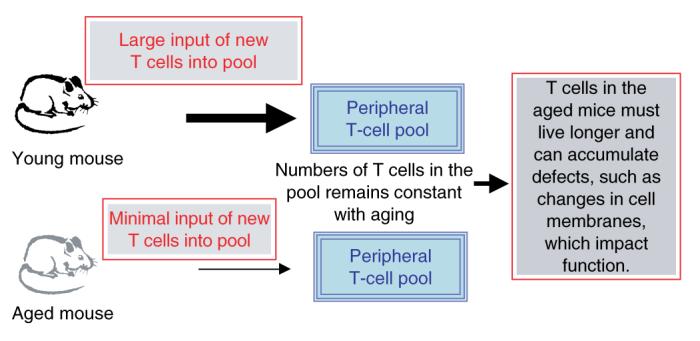
In young animals, thymic output is high, leading to a rapid turnover of T cells in the periphery. As animals age and thymic involution turnover of T cells in the periphery of aged animals must decline. This model would result in T cells in the periphery of aged animals being chronologically older than those in younger animals.
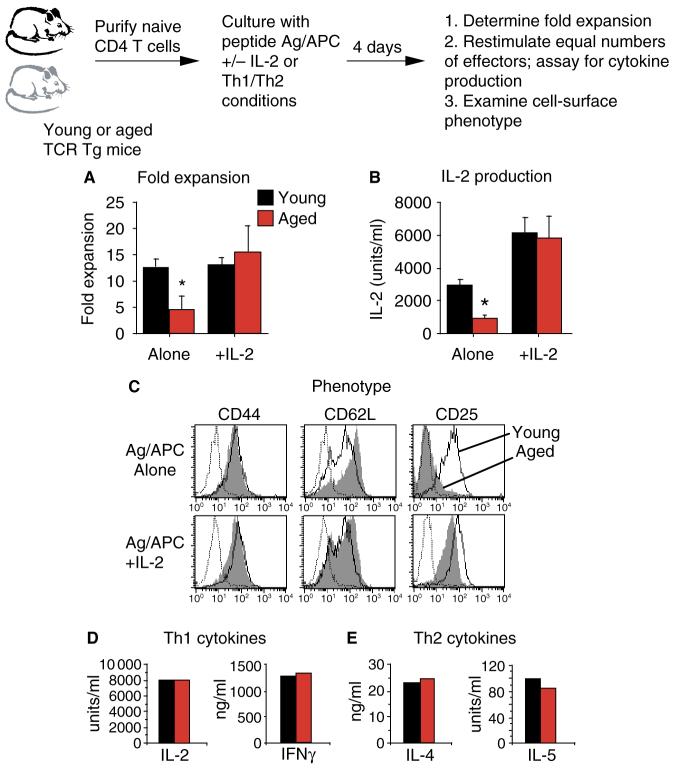
In order to specifically examine the effect of age on naive CD4+ T-cell function, we have employed a model using TCR Tg mice. AND TCR Tg mice express a Vβ3/Vα11 TCR that responds to pigeon cytochrome c (PCC) presented by I-Ek (35). In this model, the TCR Tg-expressing CD4+ T cells express a naive cell-surface (CD44lo CD62Lhi CD25neg) and functional phenotype, even in very old animals (36). This model is quite useful and allows us to directly examine and compare the responses of naive antigen-specific CD4+ T cells from young and aged animals. For these studies, naive TCR Tg CD4+ T cells were generally prepared by fluorescence-assisted cell sorting for Tg (Vβ3)-positive CD4+ T cells from young (2 to 4 months) and aged (14–19 months) mice. The APCs used for these studies were DCEK—intercellular adhesion molecule (ICAM) fibroblasts which express I-Ek, B7-1, and ICAM-1 (37). Results from in vitro studies show that naive TCR Tg CD4+ T cells from aged mice exhibit reduced expansion over a 4-day culture period when stimulated with peptide antigen and APC (Ag/APC) (Fig. 2A) or anti-CD3/anti-CD28 (36). In these studies, the fold expansion was determined by dividing the number of T cells recovered on day 4 by the input number of T cells. Naive CD4+ T cells from young mice expanded 12-fold, while those from aged mice only expanded fivefold. Fig. 2A also shows that the expansion of the aged effector population could be significantly enhanced by the addition of exogenous IL-2. When IL-2 was added, the young and the aged populations expanded 12–15-fold.
Fig. 2. In vitro responses of naive CD4+ T cells from aged mice can be enhanced by the addition of exogenous interleukin (IL)-2.
Purified populations of transgenic (Tg) CD4+ T cells from young (black) and aged (red) mice were stimulated with peptide antigen and antigen-presenting cell (APC) (DCEK—ICAM fibroblasts) with or without exogenous IL-2 (80 U/ml) for 4 days. (A) Fold expansion of each effector population was calculated after 4 days of culture. (B) Equal numbers of day 4 effectors were restimulated with Ag/APC alone. Supernatants were recovered after 24 h and assayed for the presence of IL-2. (C) Young (open) and aged (shaded) day 4 effectors were stained for CD4, Vβ3, and the indicated cell-surface markers. Flow cytometry histograms are gated on CD4+Vβ3+ cells. Dotted lines represent isotype-control staining. (D) Young and aged naive Tg CD4+ T cells were cultured under Th1 polarizing conditions for 4 days. Equal numbers of effectors were restimulated with Ag/APC alone. Supernatants were recovered after 24 h and assayed for the presence of IL-2 and interferon γ. (E) Young and aged naive Tg CD4+ T cells were cultured under Th2 polarizing conditions for 4 days. Equal numbers of effectors were restimulated with Ag/APC alone. Supernatants were recovered after 24 h and assayed for the presence of IL-4 and IL-5. For all graphs *P < 0.05 by Student’s t-test.
The ability of 4-day effectors to produce IL-2 was also examined by restimulating equal numbers of effectors with Ag/APC and assaying for IL-2 production in supernatants. In effector populations generated with Ag/APC alone, young effectors produced significantly higher levels of IL-2 compared with young effectors (Fig. 2B). Young effectors produced about 3000 U/ml of IL-2, while aged effectors only produced 1000 U/ml. In contrast, aged effector populations generated with Ag/APC plus exogenous IL-2 produced levels of IL-2 similar to young effectors upon restimulation. Because the starting populations were truly naive CD4+ T cells, none of these effector populations produced detectable levels of interferon γ (IFNγ) or IL-4 (36). The cell-surface phenotype of these young and aged effector populations was also examined by flow cytometry. Aged effector cells generated with Ag/APC alone showed reduced differentiation compared with young effectors (Fig. 2C, top panel). The aged cells did not downregulate CD62L or upregulate CD25 to the same extent as young cells. This defect in the aged effectors could be overcome by the addition of exogenous IL-2 (Fig. 2C, bottom panel). Aged effectors generated with IL-2 downregulated CD62L and upregulated CD25 similar to young effectors.
Polarized Th1 and Th2 effector populations were also generated with naive CD4+ T cells from young and aged Tg mice. Th1 effectors were generated by culturing with Ag/APC, IL-12, IL-2, and anti-IL4, while Th2 effectors were cultured with Ag/APC, IL-4, IL-2, and anti-IFNγ. Because exogenous IL-2 was provided in both polarizing conditions, the resulting young and aged effectors were quite similar. The cytokine production profiles of 4-day effectors restimulated with Ag/APC are shown in Fig. 2D,E. Young and aged Th1 effectors produced high levels of IL-2 and IFNγ, while Th2 effectors produced high levels of IL-4 and IL-5. Thus, no age-related differences were seen in either cytokine production, expansion, or cell-surface phenotype (38). As we have shown previously, significant reductions in all of these parameters were seen if exogenous IL-2 was not added to the original aged T-cell cultures during Th1 and Th2 effector generation (38).
The results presented in Fig. 2 show that the in vitro proliferation and effector differentiation of naive CD4+ T cells from aged mice can be enhanced by the addition of exogenous IL-2 to the cultures. Interestingly, IL-2 is the only cytokine that we have examined that enhances the in vitro differentiation of aged CD4+ T cells. Other cytokines that have been assayed (IL-4, IL-7, and IL-15) do not have this effect (38). Our theory is that addition of IL-2 to the cultures of aged naive CD4+ T cells enhances function by bypassing defects that occur in these cells due to decreased synapse formation. This then allows for normal (young-like) differentiation to occur in the aged effector population.
Effect of age on the in vivo function of naive CD4+ T cells
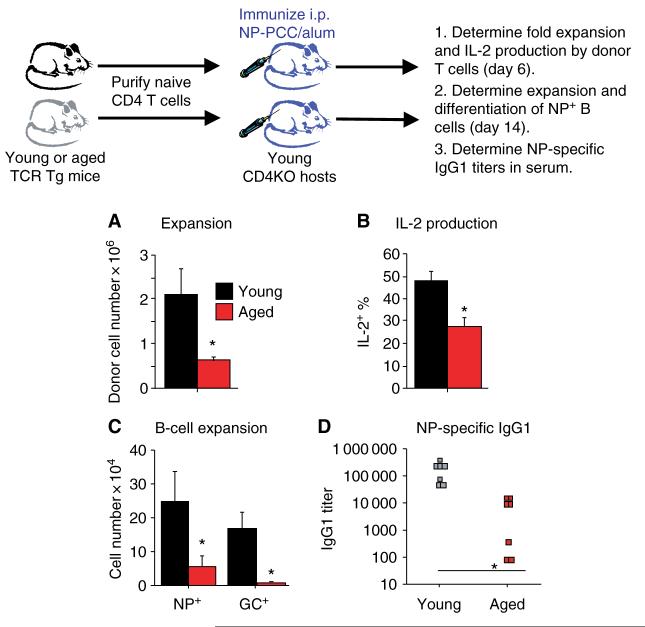
To examine the effects of age specifically on the in vivo function of naive CD4+ T cells, an adoptive transfer model was employed. This adoptive transfer system allows a direct comparison of the responses of equal numbers of antigen-specific CD4+ T cells from young and aged mice. Naive CD4+ T cells from young or aged Tg mice were transferred into young syngeneic CD4KO hosts. By using young hosts for these studies, age-related changes in other cell types, such as B cells, FDCs, and APCs, would not complicate the interpretation of the experimental results. In addition, CD4KO hosts were ideal for these studies as they exhibit no endogenous CD4+ T cells and, thus, no cognate helper activity (39). After transfer of the donor T-cell populations, the hosts were immunized with 200 μg of 4-hydroxy-3-nitrophenyl acetyl conjugated to PCC (NP-PCC) in alum. The initial expansion of the young donor T-cell population was about four times that of the aged donor population by day 6 after immunization (Fig. 3A). Furthermore, the IL-2 production by these in vivo-generated effector populations was determined by a brief restimulation with phorbol myristate acetate and ionomycin, followed by intracellular staining for IL-2 (24). Fig. 3B shows that IL-2 staining of the aged effectors was about half that seen in the young population. Therefore, much like the in vitro results, naive CD4+ T cells from aged donors exhibited reduced expansion and IL-2 production in vivo, even in a young host environment.
Fig. 3. Naive CD4+ T cells from aged mice exhibit reduced in vivo expansion and cognate helper function.
Purified populations of transgenic (Tg) CD4+ T cells from young (black) and aged (red) mice were transferred i.v. to CD4KO hosts (106 per host). Hosts were immunized with 200 μg of 4-hydroxy-3-nitrophenyl acetyl conjugated to PCC (NP-PCC)/alum i.p. on the same day. (A) On day 6, the recovery of donor T cells was determined by flow cytometry. Donor T cells were identified by CD4 Vβ3 staining. (B) On day 6, cells (spleen and lymph node) from the hosts were stimulated ex vivo with phorbol myristate acetate and ionomycin for 4 h and intracellular staining for interleukin (IL)-2 was performed. The percent of each donor CD4+ Vβ3 population staining positive for IL-2 was determined by flow cytometry. (C) On day 14, the number of NP-specific host B cells was determined by staining with NP conjugated to the fluorochrome allophycocyanin (NP-APC). The number of NP+ germinal center (GC) phenotype B cells (CD38lo PNAhi) was also determined by flow cytometry. (D) On day 14, serum was collected from each host and assayed for the presence of NP-specific immunoglobulin G1 (IgG1) antibodies. For all graphs *P < 0.05 by Student’s t-test.
Our studies have shown that immunization of aged non-Tg B10.BR mice results in reduced NP+ B-cell expansion and differentiation when compared with young mice (39). As it is difficult to dissect the impact of age on individual cell types in intact animals, we also used the adoptive transfer model to examine cognate helper function of young and aged TCR Tg CD4+ T cells. The ability of the young and aged donor CD4+ T-cell populations to provide cognate help to B cells was assessed by examining the B-cell response to the hapten NP. Adequate T-cell cognate helper activity is necessary for B-cell expansion and differentiation as well as antibody class switching and affinity maturation, all of which have been shown to decline with age (9, 17).
To examine the B-cell responses in these experiments, NP-specific B cells were identified by flow cytometry, and their expansion and differentiation to GC phenotype was determined. Results of the adoptive transfer studies show that on day 14 after immunization, the expansion of the NP+ B-cell population was fourfold higher in the hosts receiving young donor T cells when compared with those receiving aged T cells (Fig. 3C). In addition, the differentiation of the NP+ B cells to a GC phenotype (CD38lo PNAhi) was minimal in the hosts receiving aged donor T cells. Fig. 3D shows that there were also significantly higher immunoglobulin G1 (IgG1) titers in the serum of hosts receiving young donor cells. Interestingly, by day 14 after immunization, the numbers of young and aged donor CD4+ T cells recovered were equivalent; hence, differences in the numbers of helper T cells at this time point could not account for these results (39). This set of experiments indicate that the in vivo defects of aged naive CD4+ T cells, even in the young host environment, extend to reduced cognate helper activity, leading to reduced B-cell expansion and differentiation and antibody production.
Enhancement of cognate helper function
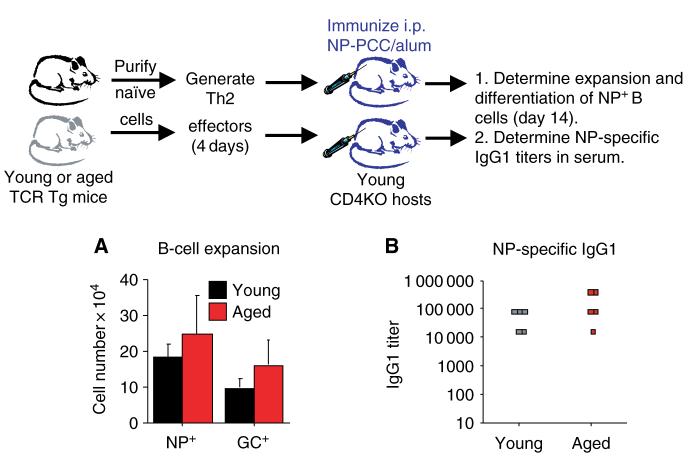
The results presented in Fig. 3 show that naive CD4+ T cells from aged mice exhibit poor cognate helper function. This poor function could potentially contribute to reduced vaccine efficacy in the elderly, especially in response to new vaccinations. Therefore, we have been examining ways to enhance the cognate function of aged T cells. As in vitro stimulation of aged T cells in the presence of IL-2 restored both expansion and differentiation, the in vivo function of these effector populations was examined. Young and aged Th2 effector populations were generated in vitro as shown in Fig. 2 and transferred into young CD4KO hosts, which were then immunized with NPPCC. Both young and aged Th2 effector populations exhibited good cognate function that led to good expansion and GC differentiation of NP+ B-cell populations by day 14 (Fig. 4A). In addition, similar levels of NP-specific IgG1 were generated (Fig. 4B) in the hosts receiving young and aged Th2 effector populations. The results of this experiment indicate that by generating highly activated Th2 effectors from aged naive cells, age-related defects could be overcome and the cognate helper function could be enhanced.
Fig. 4. In vitro-generated Th2 effectors generated from aged naive cells exhibit good cognate helper function.
Th2 effectors were generated from young (black) and aged (red) CD4+ T cells and then transferred i.v. into CD4KO hosts(107 per host). Hosts were immunized with 200 μg of 4-hydroxy-3-nitrophenyl acetyl conjugated to PCC (NPPCC)/alum i.p. on the same day. (A) On day 14, the number of NP-specific host B cells was determined by staining with NP-AC. The number of NP+ germinal center (GC) phenotype B cells (CD38lo PNAhi) was also determined by flow cytometry. (B) On day 14, serum was collected from each host and assayed for the presence of NP-specific immunoglobulin G1 (IgG1) antibodies.
Currently, the characteristics of CD4+ T-cell effectors that are required for potent cognate function remain quite unclear. As shown in Fig. 2E, young and aged Th2 effectors produce similar high levels of IL-4 and IL-5, which may play some role in cognate activity. In addition, appropriate expression of cell-surface molecules is likely to be necessary for optimal helper function and migration. Our recent work has shown that in a primary response to NP-PCC, young and aged naive CD4+ T cells migrate similarly to sites of GC formation and express similar levels of CD28, CD134 (OX40), and CXCR5 during antigen-specific responses (39). Therefore, the age-related defect in cognate function is not due to the inability to migrate to the correct location, but resides in the lack of ability to provide help once the cells have arrived. One of the most evident phenotypic differences that have been found in our model is that the aged CD4+ T cells exhibit significantly reduced CD154 (CD40L) expression compared with young T cells. This reduction is likely to have a significant impact on the function of these aged CD4+ T cells, as CD154 expression is essential for appropriate cognate function. CD154-deficient mice and humans exhibit impaired humoral immune responses, with defects in B-cell proliferation, isotype switching, GC formation, and antibody secretion (40). Thus, the ability of T cells to appropriately express CD154 is of utmost importance for B-cell responses.
Of interest to our studies, Skov and colleagues (41) have shown that expression of CD154 on CD4+ T cells was increased when IL-2 was present and was decreased when IL-2 binding was blocked. As the naive aged CD4+ T cells used in our studies produce greatly reduced levels of IL-2 upon antigenic stimulation, it is possible that this reduction is responsible for their observed reduction in CD154 expression and subsequent reduced cognate activity. In order to determine, if the addition of exogenous IL-2 to cultures of aged CD4+ T cells could enhance CD154 expression, the experiment presented in Fig. 5 was performed. Young and aged naive TCR Tg CD4+ T cells were stimulated in vitro with Ag/APC with or without the addition of IL-2. Over a period of 4 days, the expression of CD154 was examined by flow cytometry. Based on the similarities observed in the in vitro (Fig. 2) and in vivo (Fig. 3) responses to antigen in our studies, the expression of CD154 on in vitro-generated effectors was used to address this issue, as it is difficult to examine CD154 expression ex vivo. CD154 expression occurs in two phases following TCR stimulation, one very early (4 to 6 h) and one later (2 to 3 days) (42). Fig. 5A shows that the early expression of CD154 was similar in young and aged effector populations, regardless of the addition of IL-2. This finding was in contrast to CD154 expression at the later time point, which was reduced approximately 50% in the aged effector population. Importantly, addition of exogenous IL-2 to the aged cultures significantly enhanced the later phase of CD154 expression.
Fig. 5. CD154 expression and NFκB binding of aged CD4+ T cells can be enhanced by culturing with exogenous interleukin (IL)-2.
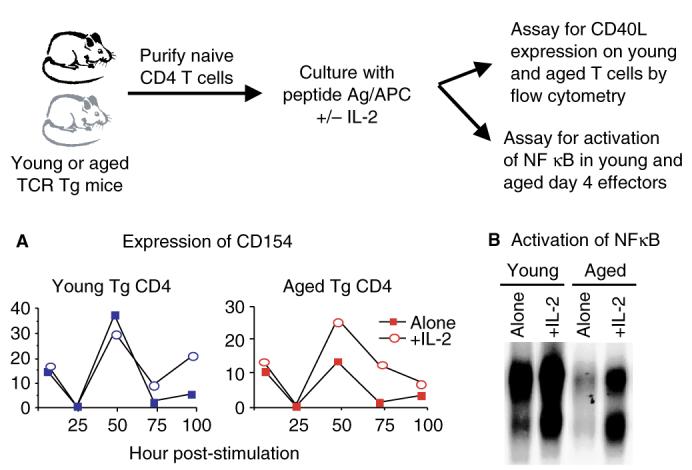
Purified populations of transgenic (Tg) CD4+ T cells from young (black) and aged (red) mice were stimulated with peptide antigen and antigen-presenting cell (DCEK—intercellular adhesion molecule fibroblasts) with or without exogenous IL-2 (80 U/ml). (A) At the indicated time points, the expression of CD154 was determined on the CD4+Vβ3+ populations by flow cytometry. (B) After 4 days of culture, equal numbers of each effector population were stimulated with anti-Vβ3/anti-CD28 for 4 h and nuclear extracts were then prepared. Electrophoretic mobility shift assays were performed using [32P]dCTP-labeled probe for NFκB.
Because CD154 expression on CD4+ T cells is dependent upon NFκB activation (43–45), we examined this transcription factor in young and aged Tg CD4+ T-cell populations. Effectors were generated with or without exogenous IL-2 for 4 days, washed, and then equal numbers were restimulated with platebound anti-Vβ3/anti-CD28 for 4 h. Electrophoretic mobility shift assays were then performed to examine the levels of NFκB binding in each population (24). As shown in Fig. 5B, young effectors generated with Ag/APC alone exhibited greater NFκB binding compared with aged effectors. Importantly, the NFκB binding in the aged effectors was enhanced greatly, if the effectors were generated in the presence of exogenous IL-2. We hypothesize that enhancement of aged effector cognate function, which occurs during generation with IL-2, is likely due in part to increased NFκB activation, resulting in increased CD154 expression.
Effect of age on host components
The results presented in Fig. 3 show that naive CD4+ T cells from aged mice exhibit significantly reduced helper function in young hosts. To determine if age-related defects existed in other components of the response, including B cells and FDCs, naive TCR Tg CD4+ T cells from young donors were transferred into young or aged CD4KO hosts. In these studies, young hosts were 2–4 months old and aged hosts were 20–24 months old. Fig. 6A shows that on day 14 postimmunization, there were no differences in the number of NP+ B cells or in the number of differentiated GC+ B cells recovered in the young and aged hosts. In both groups, substantial expansion and GC differentiation of the NP+ B cells was observed. In addition, there were no differences in the serum titers of NPspecific IgG1 antibodies in the young and aged hosts (Fig. 6B). These results indicate that aging of the host components has little to no effect on their function and on the humoral response to the hapten NP. Consequently, we propose that age-related reductions in CD4+ T-cell function are likely to account for all of the reductions in antibody production observed in this model. These results also indicate that in order to enhance the efficacy of vaccinations in the elderly, enhancement of the CD4+ T-cell response needs to be the primary goal.
Fig. 6. Young donor CD4+ T cells exhibit good cognate function in young and aged hosts.
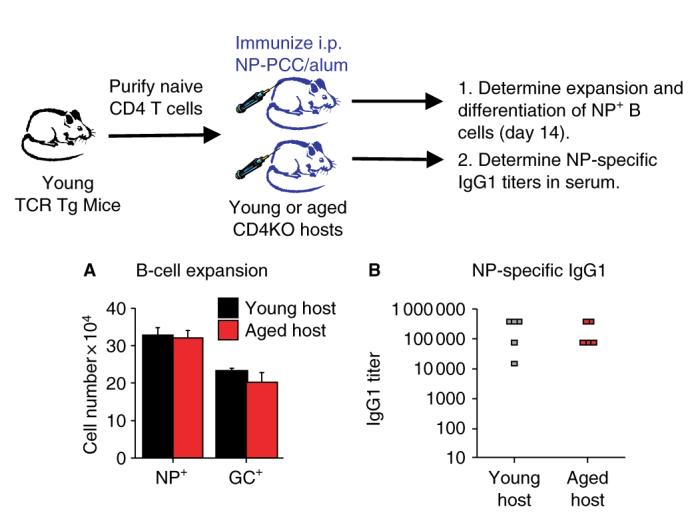
Purified populations of transgenic (Tg) CD4+ T cells from young mice were transferred i.v. to young (black) or aged (red) CD4KO hosts (106 per host). Hosts were immunized with 200 μg of 4-hydroxy-3-nitrophenyl acetyl conjugated to PCC (NP-PCC)/alum i.p. on the same day. (A) On day 14, the number of NP-specific host B cells was determined by staining with NP-APC. The number of NP+ germinal center (GC) phenotype B cells (CD38lo PNAhi) was also determined by flow cytometry. (B) On day 14, serum was collected from each host and assayed for the presence of NP-specific immunoglobulin G1 (IgG1) antibodies.
Summary
Using a TCR Tg model, we have shown that significant defects in naive CD4+ T-cell function occur with aging. These defects are apparent during in vitro responses to Ag/APC and can be overcome by the addition of exogenous IL-2. These defects are also evident in vivo and lead to reduced expansion, cytokine production, and cognate helper activity. This outcome leads to reduced antigen-specific B-cell expansion and differentiation as well as reduced IgG production. Interestingly, the defect in the cognate activity of aged CD4+ T cells could be overcome by prior in vitro effector generation in the presence of IL-2. Addition of IL-2 to cultures of aged cells enhanced NFκB binding and, subsequently, enhanced the expression of CD154, which is necessary for appropriate cognate function. Furthermore, we have shown that the function of other components of the immune system involved in humoral responses remains intact with aging and that the decline in humoral immunity with age is most likely due to reduction in CD4+ T-cell function.
References
- 1.Phair J, Kauffman A, Bjornson A, Adams L, Linnemann C. Failure to respond to influenza vaccine in the aged: correlation with B-cell number and function. J Lab Clin Med. 1978;92:822–828. [PubMed] [Google Scholar]
- 2.Musher DM, Chapman AJ, Goree A, Jonsson S, Briles D, Baughn RE. Natural and vaccine-related immunity to Streptococcus pneumoniae. J Infect Dis. 1986;154:245–256. doi: 10.1093/infdis/154.2.245. [DOI] [PubMed] [Google Scholar]
- 3.Cook JM, et al. Alterations in the human immune response to the hepatitis B vaccine among the elderly. Cell Immunol. 1987;109:89–96. doi: 10.1016/0008-8749(87)90294-2. [DOI] [PubMed] [Google Scholar]
- 4.Burns EA, Lum LG, L’Hommedieu G, Goodwin JS. Specific humoral immunity in the elderly: in vivo and in vitro response to vaccination. J Gerontol. 1993;48:B231–B236. doi: 10.1093/geronj/48.6.b231. [DOI] [PubMed] [Google Scholar]
- 5.Nicoletti C, Cerny J. A study of autologous anti-idiotypic antibody-forming cells in mice of different ages and genetic backgrounds. Cell Immunol. 1992;144:332–346. doi: 10.1016/0008-8749(92)90249-o. [DOI] [PubMed] [Google Scholar]
- 6.Nicoletti C, Borghesi-Nicoletti C, Yang XH, Schulze DH, Cerny J. Repertoire diversity of antibody response to bacterial antigens in aged mice. II. Phosphorylcholine-antibody in young and aged mice differ in both VH/VL gene repertoire and in specificity. J Immunol. 1991;147:2750–2755. [PubMed] [Google Scholar]
- 7.Nicoletti C, Yang X, Cerny J. Repertoire diversity of antibody response to bacterial antigens in aged mice. III. Phosphorylcholine antibody from young and aged mice differ in structure and protective activity against infection with Streptococcus pneumoniae. J Immunol. 1993;150:543–549. [PubMed] [Google Scholar]
- 8.Miller C, Kelsoe G. Ig VH hypermutation is absent in the germinal centers of aged mice. J Immunol. 1995;155:3377–3384. [PubMed] [Google Scholar]
- 9.Zheng B, Han S, Takahashi Y, Kelsoe G. Immunosenescence and germinal center reaction. Immunol Rev. 1997;160:63–77. doi: 10.1111/j.1600-065x.1997.tb01028.x. [DOI] [PubMed] [Google Scholar]
- 10.Manz RA, Thiel A, Radbruch A. Lifetime of plasma cells in the bone marrow. Nature. 1997;388:133–134. doi: 10.1038/40540. [DOI] [PubMed] [Google Scholar]
- 11.Tsiagbe VK, Inghirami G, Thorbecke GJ. The physiology of germinal centers. Crit Rev Immunol. 1996;16:381–421. [PubMed] [Google Scholar]
- 12.Manser T. Textbook germinal centers? J Immunol. 2004;172:3369–3375. doi: 10.4049/jimmunol.172.6.3369. [DOI] [PubMed] [Google Scholar]
- 13.MacLennan IC. Germinal centers. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 14.Smith KG, Light A, Nossal GJ, Tarlinton DM. The extent of affinity maturation differs between the memory and antibody-forming cell compartments in the primary immune response. EMBO J. 1997;16:2996–3006. doi: 10.1093/emboj/16.11.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacob J, Kelsoe G, Rajewsky K, Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991;354:389–392. doi: 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- 16.Berek C, Berger A, Apel M. Maturation of the immune response in germinal centers. Cell. 1991;67:1121–1129. doi: 10.1016/0092-8674(91)90289-b. [DOI] [PubMed] [Google Scholar]
- 17.Song H, Price PW, Cerny J. Age-related changes in antibody repertoire: contribution from T cells. Immunol Rev. 1997;160:55–62. doi: 10.1111/j.1600-065x.1997.tb01027.x. [DOI] [PubMed] [Google Scholar]
- 18.Yang X, Stedra J, Cerny J. Relative contribution of T and B cells to hypermutation and selection of the antibody repertoire in germinal centers of aged mice. J Exp Med. 1996;183:959–970. doi: 10.1084/jem.183.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller RA. The aging immune system: primer and prospectus. Science. 1996;273:70–74. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- 20.Grossmann A, Maggio-Price L, Jinneman JC, Rabinovitch PS. Influence of aging on intracellular free calcium and proliferation of mouse T-cell subsets from various lymphoid organs. Cell Immunol. 1991;135:118–131. doi: 10.1016/0008-8749(91)90259-e. [DOI] [PubMed] [Google Scholar]
- 21.Grossmann A, et al. Activation of murine T-cells via phospholipase-C gamma 1-associated protein tyrosine phosphorylation is reduced with aging. J Gerontol a Biol Sci Med Sci. 1995;50:B205–B212. doi: 10.1093/gerona/50a.4.b205. [DOI] [PubMed] [Google Scholar]
- 22.Garcia GG, Miller RA. Differential tyrosine phosphorylation of zeta chain dimers in mouse CD4 T lymphocytes: effect of age. Cell Immunol. 1997;175:51–57. doi: 10.1006/cimm.1996.1040. [DOI] [PubMed] [Google Scholar]
- 23.Tamir A, Eisenbraun MD, Garcia GG, Miller RA. Age-dependent alterations in the assembly of signal transduction complexes at the site of T cell/APC interaction. J Immunol. 2000;165:1243–1251. doi: 10.4049/jimmunol.165.3.1243. [DOI] [PubMed] [Google Scholar]
- 24.Haynes L, Eaton SM, Burns EM, Rincon M, Swain SL. Inflammatory cytokines overcome age-related defects in CD4 T cell responses in vivo. J Immunol. 2004;172:5194–5199. doi: 10.4049/jimmunol.172.9.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ernst DN, et al. Differences in the expression profiles of CD45RB, Pgp-1 and 3G11 membrane antigens and the pattern of lymphokine secretion by splenic CD4+ T cells from young and aged mice. J Immunol. 1990;145:1295–1302. [PubMed] [Google Scholar]
- 26.Lerner A, Yamada T, Miller RA. Pgp-1hi T lymphocytes accumulate with age in mice and respond poorly to concanavalin A. Eur J Immunol. 1989;19:977–982. doi: 10.1002/eji.1830190604. [DOI] [PubMed] [Google Scholar]
- 27.Nagelkerken L, Hertogh-Huijbregts A, Dobber R, Drager A. Age-related changes in lymphokine production related to a decreased number of CD45RBhi CD4 T cells. Eur J Immunol. 1991;21:273–278. doi: 10.1002/eji.1830210206. [DOI] [PubMed] [Google Scholar]
- 28.Rogers PR, Dubey C, Swain SL. Qualitative changes accompany memory T cell generation: faster, more effective responses at lower doses of antigen. J Immunol. 2000;164:2338–2346. doi: 10.4049/jimmunol.164.5.2338. [DOI] [PubMed] [Google Scholar]
- 29.Garcia GG, Miller RA. Single-cell analyses reveal two defects in peptide-specific activation of naive T cells from aged mice. J Immunol. 2001;166:3151–3157. doi: 10.4049/jimmunol.166.5.3151. [DOI] [PubMed] [Google Scholar]
- 30.Garcia GG, Miller RA. Age-dependent defects in TCR-triggered cytoskeletal rearrangement in CD4+ T cells. J Immunol. 2002;169:5021–5027. doi: 10.4049/jimmunol.169.9.5021. [DOI] [PubMed] [Google Scholar]
- 31.Garcia GG, Miller RA. Age-related defects in CD4+ T cell activation reversed by glycoprotein endopeptidase. Eur J Immunol. 2003;33:3464–3472. doi: 10.1002/eji.200324310. [DOI] [PubMed] [Google Scholar]
- 32.Berzins SP, Boyd RL, Miller JF. The role of the thymus and recent thymic migrants in the maintenance of the adult peripheral lymphocyte pool. J Exp Med. 1998;187:1839–1848. doi: 10.1084/jem.187.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li L, et al. Cellular mechanism of thymic involution. Scand J Immunol. 2003;57:410–422. doi: 10.1046/j.1365-3083.2003.01206.x. [DOI] [PubMed] [Google Scholar]
- 34.Lohmiller JJ, Roellich KM, Toledano A, Rabinovitch PS, Wolf NS, Grossmann A. Aged murine T-lymphocytes are more resistant to oxidative damage due to the predominance of the cells possessing the memory phenotype. J Gerontol A Biol Sci Med Sci. 1996;51:B132–B140. doi: 10.1093/gerona/51a.2.b132. [DOI] [PubMed] [Google Scholar]
- 35.Kaye J, Hsu M-L, Sauron M-E, Jameson SC, Gascoigne NRJ, Hedrick SM. Selective development of CD4+ T cells in transgenic mice expressing a class II MHC-restricted antigen receptor. Nature. 1989;341:746–749. doi: 10.1038/341746a0. [DOI] [PubMed] [Google Scholar]
- 36.Linton P-J, Haynes L, Klinman NR, Swain SL. Antigen independent changes in CD4 T cells with aging. J Exp Med. 1996;184:1891–1900. doi: 10.1084/jem.184.5.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dubey C, Croft M, Swain SL. Costimulatory requirements of naive CD4+ T cells. ICAM-1 or B7–1 can costimulate naive CD4 T cell activation but both are required for optimum response. J Immunol. 1995;155:45–57. [PubMed] [Google Scholar]
- 38.Haynes L, Linton P-J, Eaton SM, Tonkonogy SL, Swain SL. IL-2, but not other common γ chain (γc)-binding cytokines, can reverse the defect in generation of CD4 effector T cells from naive T cells of aged mice. J Exp Med. 1999;190:1013–1023. doi: 10.1084/jem.190.7.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eaton SM, Burns EM, Kusser K, Randall TD, Haynes L. Age-related defects in CD4 T cell cognate helper function lead to reductions in humoral responses. J Exp Med. 200:1613–1622. doi: 10.1084/jem.20041395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foy TM, Aruffo A, Bajorath J, Buhlmann JE, Noelle RJ. Immune regulation by CD40 and its ligand GP39. Annu Rev Immunol. 1996;14:591–617. doi: 10.1146/annurev.immunol.14.1.591. [DOI] [PubMed] [Google Scholar]
- 41.Skov S, Bonyhadi M, Odum N, Ledbetter JA. IL-2 and IL-15 regulate CD154 expression on activated CD4 T cells. J Immunol. 2000;164:3500–3505. doi: 10.4049/jimmunol.164.7.3500. [DOI] [PubMed] [Google Scholar]
- 42.Lee BO, Haynes L, Eaton SM, Swain SL, Randall TD. The biological outcome of CD40 signaling is dependent on the duration of CD40 ligand expression: reciprocal regulation by interleukin (IL)-4 and IL-12. J Exp Med. 2002;196:693–704. doi: 10.1084/jem.20020845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mendez-Samperio P, Ayala H, Vazquez A. NF-kappaB is involved in regulation of CD40 ligand expression on Mycobacterium bovis bacillus Calmette-Guerin-activated human T cells. Clin Diagn Lab Immunol. 2003;10:376–382. doi: 10.1128/CDLI.10.3.376-382.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Srahna M, et al. NF-kappaB is involved in the regulation of CD154 (CD40 ligand) expression in primary human T cells. Clin Exp Immunol. 2001;125:229–236. doi: 10.1046/j.1365-2249.2001.01601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smiley ST, Csizmadia V, Gao W, Turka LA, Hancock WW. Differential effects of cyclosporine A, methylprednisolone, mycophenolate, and rapamycin on CD154 induction and requirement for NFkappaB: implications for tolerance induction. Transplantation. 2000;70:415–419. doi: 10.1097/00007890-200008150-00005. [DOI] [PubMed] [Google Scholar]