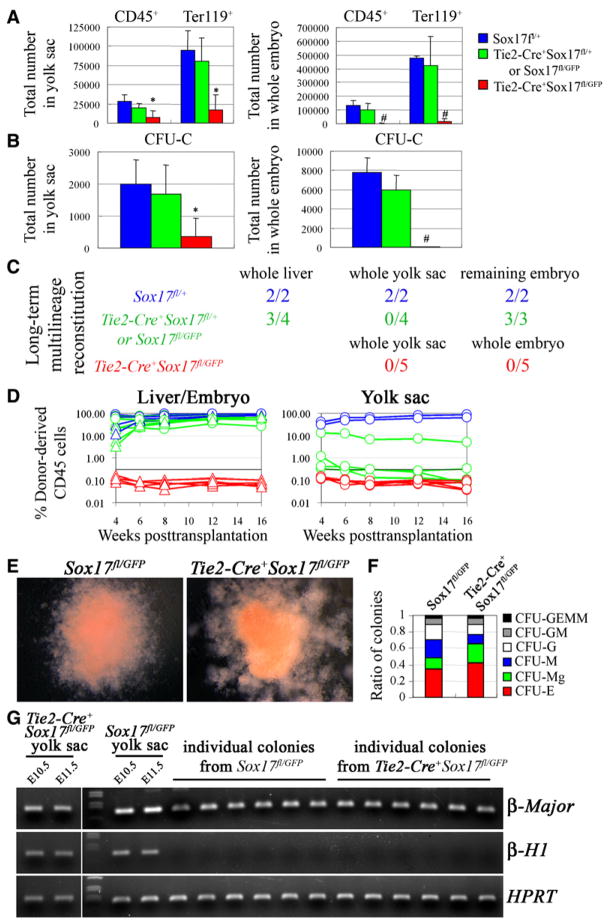
Figure 5. Conditional Deletion of Sox17 Using Tie2-Cre Leads to a Failure to Generate or Maintain HSCs.
The numbers of CD45+ or Ter119+ cells (A) and colony-forming progenitors (CFU-C; B) were dramatically reduced in the yolk sac and embryo of E11.5 Tie2-Cre+Sox17fl/GFP mice as compared to littermate controls (*, p < 0.05; #, p < 0.01; four independent experiments).
(C) Irradiated wild-type mice were transplanted with unfractionated fetal liver, yolk sac, or remaining embryo cells from single E12.5 Tie2-Cre+Sox17fl/GFP embryos or littermate controls. The livers of Tie2-Cre+Sox17fl/GFP embryos were so hypocellular and pale that the entire embryos were dissociated and transplanted, rather than just fetal liver. Numbers indicate the fraction of recipient mice that were long-term multilineage reconstituted by donor cells.
(D) While control cells usually gave long-term multilineage reconstitution (blue and green lines), we never detected any reconstitution above background (0.3%) from Tie2-Cre+Sox17fl/GFP cells (red lines).
(E) CFU-GEMM colonies from E11.5 Sox17fl/GFP and Tie2-Cre+Sox17fl/GFP yolk sac were similar in size and appearance.
(F) Sox17 deletion also did not affect the proportions of colony types, though the absolute number of all colonies was reduced (three independent experiments).
(G) E11.5 Tie2-Cre+Sox17fl/GFP and Sox17fl/GFP yolk sac cells expressed embryonic (β-H1) and adult (β-major) hemoglobin in vivo. In culture, all colonies from Sox17fl/GFP yolk sac (19/19) and Tie2-Cre+Sox17fl/GFP yolk sac (20/20) expressed adult hemoglobin (β-major). PCR on genomic DNA from individual colonies demonstrated that 95% of Tie2-Cre+Sox17fl/GFP colonies (19/20) had completely recombined Sox17 (data not shown).