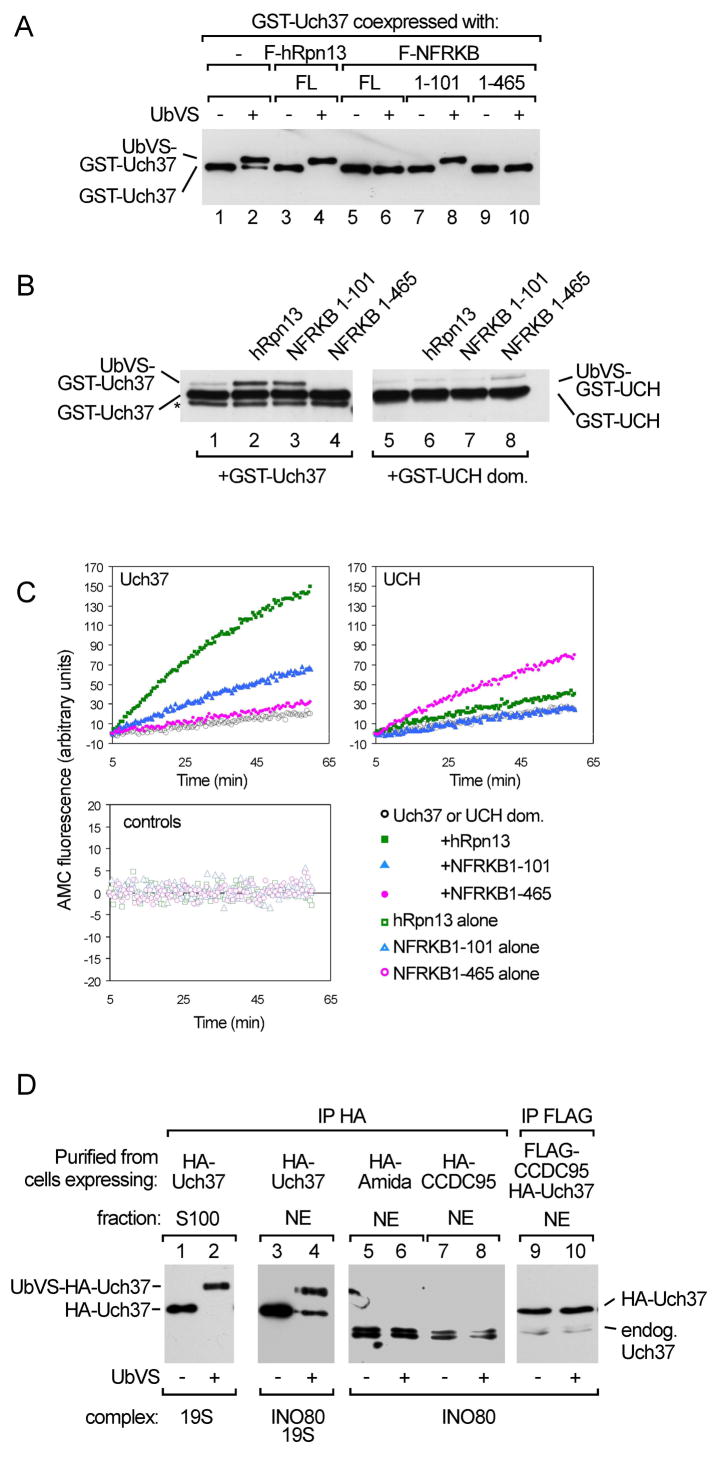
Figure 3.
Regulation of Uch37 activity by interaction with NFRKB or incorporation into hINO80. (A) GST-Uch37 alone or in complex with Flag-tagged hRpn13, NFRKB, or NFRKB fragments expressed in insect cells, affinity purified using glutathione-agarose (lanes 1 and 2) or anti-Flag agarose (lanes 3–10), incubated with or without 2 μM UbVS at 37 °C for 1 h, and analyzed by anti-GST immunoblotting. For a coomassie-stained gel of the proteins, see Figure S2. (B) 0.1 μM GST-Uch37 or GST-UCH were pre-incubated for 1 h with or without 0.5 μM hRpn13, N101, or N465 at 21°C; 0.25 μM UbVS was then added. After 30 min, reaction products were analyzed by anti-GST immunoblotting. The asterisk denotes a degradation product. (C) UbAMC hydrolysis monitored by AMC fluorescence. As indicated, reactions contained 2.5 nM full-length GST-Uch37, 2.5 nM GST-UCH, 12.5 nM hRpn13, 12.5 nM N101, or 6.25 nM N465. A coomassie-stained gel of recombinant proteins is shown in Figure S3. (D) hINO80-associated Uch37 did not react with UbVS. Affinity-purified HA-Uch37 from cytoplasmic (lanes 1 and 2) or nuclear (lanes 3 and 4) fractions of HEK293 cells was incubated with or without 2 μM UbVS at 37 °C for 1 h. Lanes 5–10, hINO80 affinity-purified from stable cell lines expressing HA-tagged hINO80 subunit Amida, HA-tagged CCDC95, or Flag-tagged CCDC95 was incubated with or without UbVS. Reaction products were detected by anti-Uch37 immunoblotting.