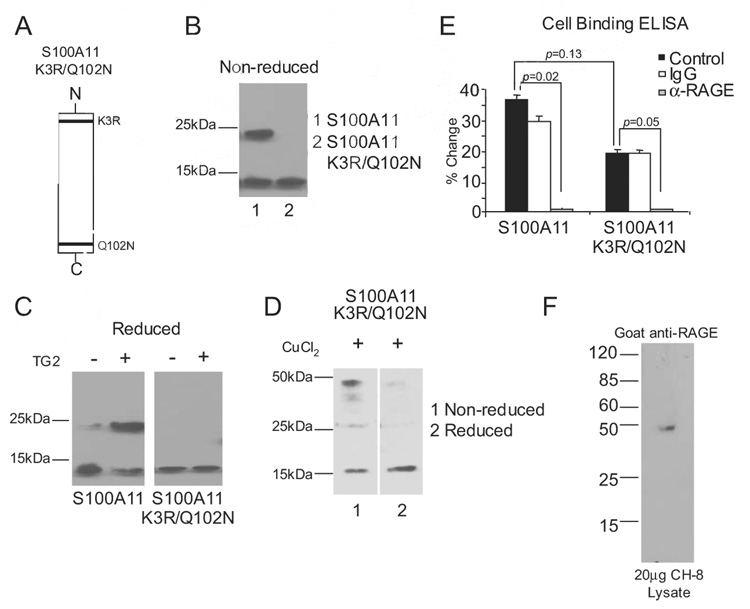
FIGURE 4.
Characterization of the S100A11 K3R/Q102N mutant. A, Graphic representation of the TG2 transamidation substrate sites of K3R and Q102N in the S100A11 protein. B, Both TG2 transamidation sites in recombinant human S100A11 were altered by site-directed mutagenesis to generate S100A11 K3R/Q102N. After purification, S100A11 and S100A11 K3R/Q102N proteins were isolated. S100A11 was separated by SDS-PAGE under nonreducing conditions and Western blotting performed. The wild-type S100A11 was detected as both monomeric (~12 kDa) and dimeric (~24 kDa), but the TG double transamidation site mutant was only detected in monomeric form. C, A total of 1 µg of S100A11 and S100A11 K3R/Q102N were incubated with 100 ng/ml TG2 for 2 h at 37°C, and SDS-PAGE under reducing conditions and Western blotting for S100A11 were performed. Data are representative of four different experiments. D, A total of 1 µg of S100A11 K3R/Q102N was incubated with 6 µM CuCl2 for 1 h at 37°C. SDS-PAGE under nonreducing and reducing conditions and Western blotting were performed for S100A11. Data are representative of three different experiments. E, Cell-associated RAGE binding ELISA was performed as described in Materials and Methods. Results are presented as the percentage of change compared with wells not receiving S100A11 or S100A11 K3R/Q102N, with data pooled from four experiments. F, A total of 20 µg of human chondrocytic CH-8 cell lysates was separated by SDS-PAGE under reducing conditions, and Western blotting was performed for RAGE using goat polyclonal Abs.