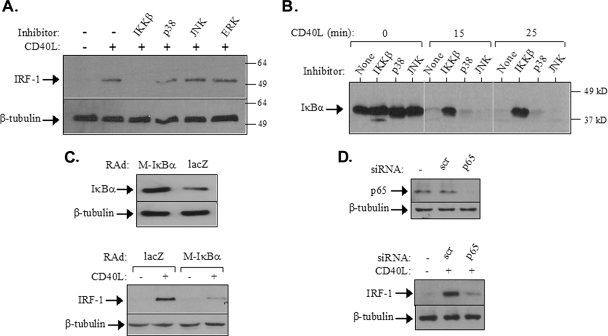
FIG. 3.
CD40 ligation induces IRF-1 expression via NF-κB. (A) EJ cells were pretreated with chemical inhibitors targeting IKKβ (34), JNK (SP600125) (2), p38 (SB203580) (36), or MEK, the ERK kinase (PD098059) (11), at a final concentration of 20 μM and then exposed to CD40L for 2 h prior to lysis and the assessment of IRF-1 expression by immunoblotting. Reblotting with anti-β-tubulin was used to evaluate the levels of protein loading. Numbers to the right are molecular sizes in kilodaltons. +, present; −, absent. (B) The IKKβ but not the JNK or p38 inhibitor blocks the CD40L-induced IκBα degradation. EJ cells were pretreated with the chemical inhibitors listed in the legend to panel A and then stimulated with 0.5 μg of CD40L/ml for the indicated times. Cell lysates were then evaluated for the levels of IκBα expression by immunoblotting. (C) The overexpression of transdominant IκBα suppresses CD40-mediated IRF-1 upregulation. EJ cells were infected with a RAd expressing either Μ-IκBα, carrying a Ser32→Ala/Ser36→Ala double mutation, or as a control, lacZ at a multiplicity of infection of 50. (Lower panel) Thirty-six hours postinfection, cells were either treated with CD40L for 2 h or left untreated and then examined for the expression of IRF-1 or β-tubulin by immunoblotting. (Upper panel) Cell lysates from untreated infected cultures were also assessed for IκBα or tubulin levels. (D) The RNAi-mediated knockdown of p65 (RelA) NF-κB inhibits the effects of CD40L on IRF-1 upregulation. EJ cells were transfected with siRNA against p65 or with scramble siRNA (scr) as a control as described in Materials and Methods. (Upper panel) Lysates were evaluated for p65 or tubulin levels by immunoblotting. (Lower panel) Parallel cultures were treated with CD40L for 2 h and then examined for the expression of IRF-1 or β-tubulin by immunoblotting.