FIG. 5.
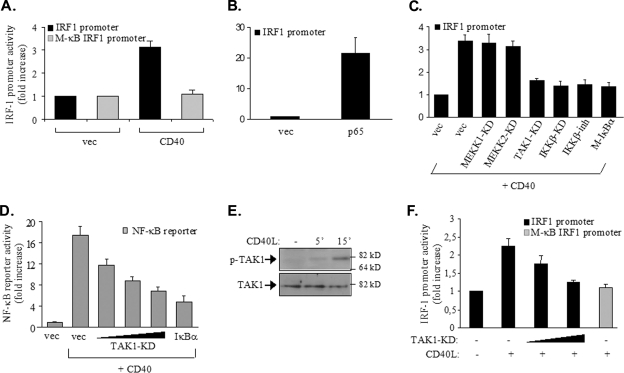
p65 NF-κB regulates the activity of the IRF-1 promoter in CD40-stimulated tumor cells. (A) The NF-κB binding site of the IRF-1 promoter is required for promoter activity in response to CD40: HEK 293 cells (105) were transfected with 50 ng of a luciferase reporter construct containing approximately 600 bp of the IRF-1 promoter or a reporter construct in which the NF-κB binding site was mutated to impair the binding of NF-κB (M-κB IRF1 promoter), according to the results shown in Fig. 4A. Cells were cotransfected with 500 ng of a CD40 expression vector (CD40) or a control empty plasmid (vec). The transfection efficiency was monitored by using a Renilla reporter. Luciferase and Renilla activities were assessed at 30 h posttransfection and expressed as the increase in reporter activity (measured as the ratio of luciferase to Renilla values) in relation to the activity in cells transfected with the control vector expressing no CD40. The mean values ± standard deviations (SD) from three independent experiments are shown. (B) The ectopic expression of p65 induces IRF-1 transactivation: HEK 293 cells were transfected with 50 ng of the IRF-1-luciferase reporter construct, 50 ng of a Renilla reporter, and 100 ng of p65 RelA, and reporter activities were assessed as described in the legend to panel A. The y axis depicts the increase (n-fold) in IRF-1 promoter activity. (C) TAK1 and IKKβ participate in CD40-mediated IRF-1 transactivation: cells were transfected with reporter plasmids as described in the legend to panel A, 250 ng of CD40, and 100 ng of kinase-dead (KD) mutants of TAK1, MEKK1, MEKK2, or IKKβ prior to lysis and the assessment of reporter activities. In parallel experiments, cells transfected with CD40 and reporter constructs were treated with an IKKβ chemical inhibitor (IKKβ-inh) (34) for 6 h prior to the assessment of reporter activity. As a control, the effects of Μ-IκBα, carrying a Ser32→Ala/Ser36→Ala double mutation, on CD40-induced IRF-1 transactivation were determined. Mean values ± SD from at least four independent experiments are shown. The y axis depicts the increase (n-fold) in IRF-1 promoter activity. (D) TAK1 participates in CD40-mediated NF-κB transactivation: HEK 293 cells were transfected with 50 ng of an NF-κB-luciferase reporter construct, 50 ng of a Renilla reporter construct, 250 ng of CD40, and 50, 100, or 200 ng of kinase-dead mutant form of TAK1 or, as a control, 100 ng of transdominant IκBα prior to lysis and the assessment of reporter activities as described in the legend to panel A. Mean values ± SD from three independent experiments are shown. Immunoblotting of lysates from the transfected cultures confirmed equal levels of CD40 expression (data not shown). (E) CD40 ligation induces the phosphorylation of TAK1: EJ cells were stimulated with 0.5 μg of CD40L/ml for the indicated times or left untreated (−) prior to lysis and the analysis of the levels of Thr187-phosphorylated, active TAK1 (p-TAK1) or total (phosphorylated and unphosphorylated) TAK1 by immunoblotting. The data shown are representative of results from three independent experiments. 5′, 5 min. (F) EJ cells were transfected with 500 ng of each Renilla reporter and a luciferase reporter containing either the IRF-1 promoter or the IRF-1 M-κB form of the promoter, along with 200 or 500 ng of kinase-dead TAK1. Cells were treated with CD40L for 6 h prior to lysis and the assessment of reporter activities as described in the legend to panel A. Mean values ± SD from three independent experiments are shown. +, present; −, absent.