FIG. 7.
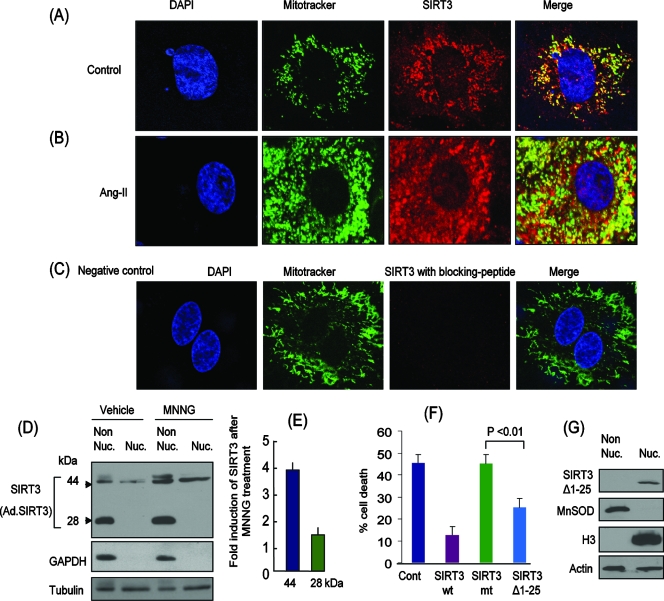
Overexpressed SIRT3 is localized in the cytoplasm and mitochondria as well as the nuclei of cardiomyocytes. Representative confocal microscopic images of cardiomyocytes infected with ad.hSIRT3 vector and stained with anti-SIRT3 antibody (SC49744) (red). Mitotracker and DAPI staining was utilized for localization of mitochondria and nuclei, respectively. (A) Control cells treated with vehicle. (B) Cells treated with Ang-II (5 μM) for 48 h. Note the induction of SIRT3 in both the mitochondria and nuclei of the cell. (C) Negative control in which a specific blocking peptide was used to validate the specificity of SIRT3 antibody. (D) Cells were overexpressed with ad.hSIRT3 and treated with vehicle or MNNG (500 μM) for 6 h. Subcellular fractions (nuclear and nonnuclear) were prepared and analyzed for the expression of SIRT3 by Western analysis (SIRT3 antibody PAB-11098). GAPDH was utilized as a cytosolic marker, and tubulin was used as a loading control. (E) Quantification of SIRT3 induction after MNNG treatment of cells. Note induction of both the 44- and 28-kDa forms of SIRT3 after genotoxic stress. (F) SIRT3Δ1-25 deletion mt lacking the mitochondrial import signal is effective in protecting cells from MNNG-mediated cell death. HeLa cells were overexpressed with wt SIRT3, mt SIRT3 (catalytically inert), or SIRT3Δ1-25. Cells were treated with MNNG (500 μM), and cell death was monitored 3 h later. Note significantly reduced cell death in SIRT3Δ1-25-expressing cells compared to that of cells overexpressing catalytically inert protein. Cont, control. Values are means ± standard errors of four separate experiments. (G) Localization of SIRT3Δ1-25 deletion mt in the nucleus but not in the nonnuclear fraction.