FIG. 5. GRP-75, a stress response protein undergoes phosphorylation in cells treated with Ang II and it is localized to the cytoplasm.
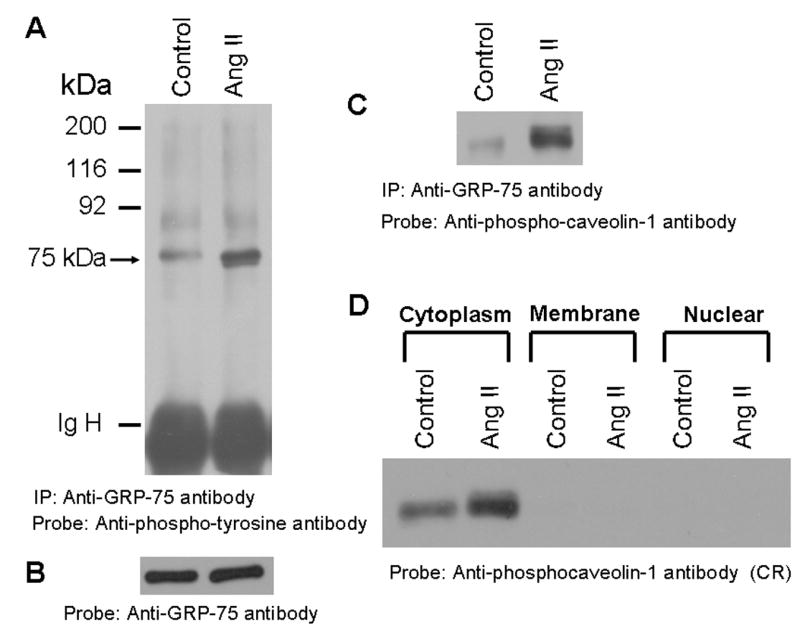
A, Phospho-tyrosine blot of immunoprecipitated GRP-75. Serum straved cells were left untreated, or treated with Ang II for 15 min (100 nM). Cell lysates were prepared and immunoprecipitated (IP) with anti-GRP-75 antibody, immunocomplexes were run on an 8% SDS-polyacrylamide gel and probed with anti-phosphotyrosine antibody. B, the blot in A was stripped and reprobed with anti-GRP-75 antibody. C, the blot representing A was stripped and reprobed with anti-phospho-caveolin-1 antibody. D, shows that the 75 kDa protein is localized to the cytoplasm. Serum starved cells were untreated or treated with Ang II for 15 min (100 nM) and lysed, and then membrane, cytoplasm and nuclear fractions were prepared. 15 μg of each sample were run on a 8% SDS-polyacrylamide gel and immunoblotted with phospho-specific anti-caveolin-1 antibody cross-reactive to the 75 kDa protein (phosphorylated GRP-75). These blots are representative of three independent experiments. IgH, Immunoglobulin heavy chain.
