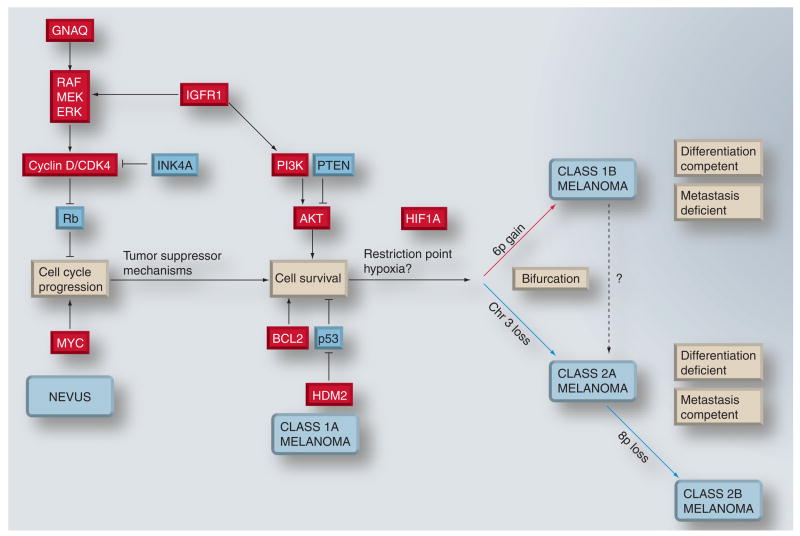
Figure 3. Provisional model for malignant progression in uveal melanoma.
Early events probably include those that lead to cell-cycle deregulation. Such mutations trigger tumor suppressor mechanisms that lead to apoptosis and/or senescence. In most cases, these mechanisms succeed in either eliminating the tumor or arresting it at the stage of a benign nevus. The tumor can continue to progress if these mechanisms can be overcome by further mutations that block apoptosis and promote survival. Eventually, this progression leads to a lesion that would correspond clinically to uveal melanoma. Early uveal melanomas are relatively uniform in their gene-expression signature, with minimal aneuploidy (class 1A). Tumor progression continues as additional genetic lesions accumulate, and tumors either undergo gain of chromosome 6p and retain a class 1 gene-expression signature (class 1B), or they acquire a class 2 gene-expression signature, usually associated with loss of chromosome 3. As class 2A tumors acquire further genetic lesions and genomic instability, they can progress to a class 2B stage, associated with 8p loss and more rapid onset of metastasis. Class 1 tumors exhibit melanocytic differentiation and a low metastatic risk, whereas class 2 tumors show defective differentiation and high metastatic risk. Chromosomal gain and gene upregulation are indicated in red. Chromosomal loss and gene downregulation are indicated in blue.