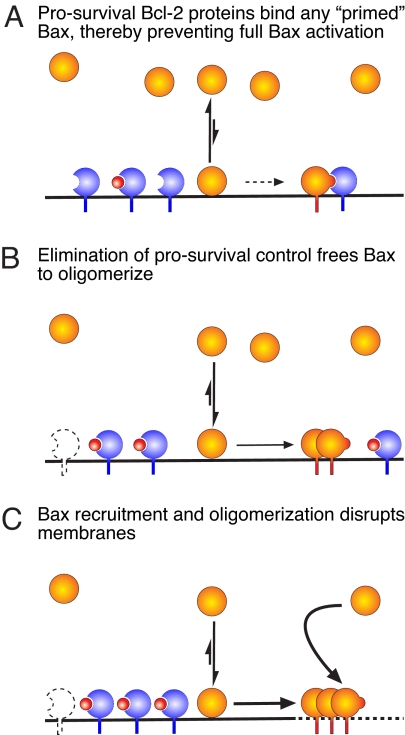
Fig. 6.
Model for Bax regulation. (A) In healthy cells, Bax (orange) is predominantly cytosolic but is in equilibrium with minor populations on the mitochondrial membrane. Posttranslational modifications, physical stimuli, and certain BH3-only proteins (e.g., tBid) may enhance its translocation to membranes. However, any membrane-bound Bax conformers with the BH3 domain exposed (“primed Bax”) are sequestered by available prosurvival Bcl-2 proteins (blue), preventing full Bax activation and apoptosis. (B) Upon cellular stress, the capacity of the prosurvival proteins to bind Bax can be overwhelmed because of their inactivation by BH3-only proteins (red), or their depressed synthesis or degradation (prosurvival ghost). Left unchecked, the membrane-bound Bax can begin to self-associate and oligomerize. (C) Further translocation of Bax molecules, perhaps by direct recruitment from the cytosol, stimulates the Bax oligomerization that is thought to permeabilize the outer mitochondrial membrane, leading to activation of the caspases that dismantle the cell.