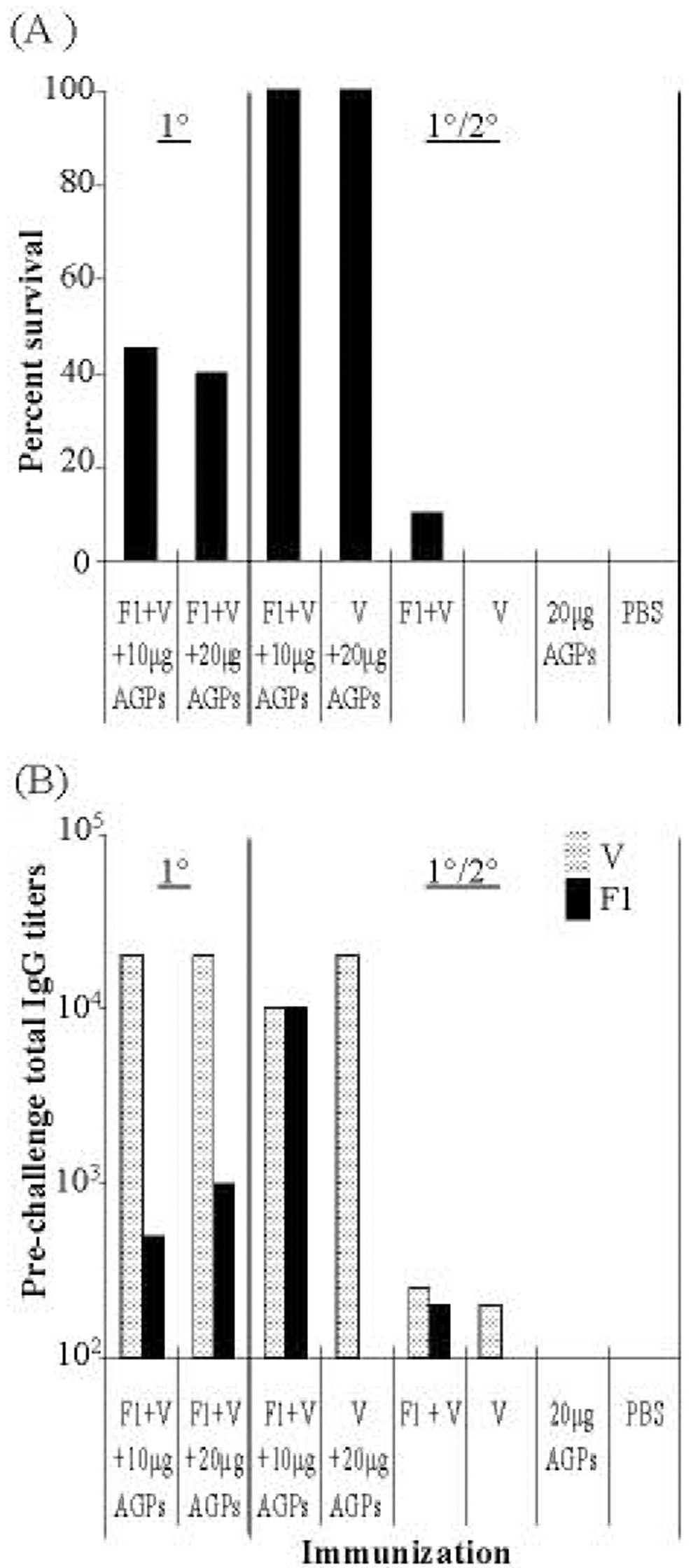
Fig. 2. Vaccine protection against pneumonic plague is enhanced by a 2° application.
Groups of BALB/c mice (n = 8) that received a 1° i.n. vaccination alone or a 1° and a 2° i.n. vaccination 10 days later were compared. The vaccine consisted of combinations of 1 µg F1, 3 µg V, and 5 -10 µg each CRX-524 and CRX-527. Forty-five days after 1° vaccination, all animals were challenged with 100LD50s Y. pestis CO92 and survival measured at 14 days post challenge. Actual i.n. challenge doses were 1.7 × 106 CFU for 1° vaccine group; 2.4 × 106 CFU for the 1° and 2° vaccine group; and 3.0 × 106 CFU for the 1° and 2° V-antigen alone group.
A: Mice received a 1° i.n. vaccine on day 0 or a 1° and 2° vaccine on day 0 and day 10; control groups received 1° and 2° i.n. (F1 + V), (V alone), (AGPs alone), or (PBS). Only those animals receiving the 1°/2° vaccine were completely protected (P < 0.0001)
B: Pooled sera total IgG titers against V  and F1
and F1  were measured in immunized animals 35 days after 1° vaccination.
were measured in immunized animals 35 days after 1° vaccination.