Summary
Salmonella pathogenesis is dependent on its ability to invade and replicate within host cells. Following invasion the bacteria remain within a modified phagosome known as the Salmonella-containing vacuole (SCV), within which they will survive and replicate. Invasion and SCV biogenesis are dependent on two Type III Secretion Systems, T3SS1 & T3SS2, which are used to translocate distinct cohorts of bacterial effector proteins into the host cell. Elucidating the roles of individual effector proteins in SCV biogenesis has proven difficult but several distinct themes are now emerging and it is apparent that SCV biogenesis is an extremely dynamic process involving; extensive membrane remodeling, interactions with the endolysosomal pathway, actin rearrangements and microtubule-based movement and tubule extension.
Introduction
The Salmonella enterica species is made up of a group of over 2,000 highly related serovars, which are 95–99% identical at the genetic level. Nevertheless, S. enterica serovars have different host specificities and cause a variety of different diseases in man and other animals. Typhoid fever, a severe systemic disease caused by the human restricted serovar Typhi, annually afflicts approximately 21 million people worldwide, almost all in the developing world. In contrast, non-typhoidal Salmonellosis, a usually self-limiting gastrointestinal infection, can be caused by a number of serovars and is endemic throughout the world. Serovar Typhimurium is one of the serovars most often associated with gastroenteritis in man but induces a typhoid-like systemic disease in susceptible mice. Almost all of what we know about the molecular basis of Salmonella-host cell interactions comes from studies using Typhimurium and cultured mammalian cells.
Salmonella virulence is dependent on the ability of the bacterium to invade non-phagocytic host cells and then to survive and replicate within a modified phagosome known as the Salmonella-containing vacuole (SCV), although it should be noted that the factors determining systemic vs localized infection remain undetermined. Both invasion and intracellular survival/replication are mediated by chromosomally encoded virulence genes, which are clustered on several Salmonella pathogenicity islands. In particular two Type III secretion systems, T3SS1 and T3SS2, are used to translocate cohorts of bacterial effector proteins directly into host cells where they can manipulate host cell processes such as membrane trafficking or signal transduction. The two systems have different functions, T3SS1 is required for invasion of non-phagocytic cells whereas T3SS2 is implicated in intracellular survival and biogenesis of the SCV, and are expressed at different times. Recent studies have revealed a hitherto unexpected overlap in function of the two T3SS, particularly with respect to the role of T3SS1 in SCV biogenesis. Here we summarize and discuss what is known about SCV biogenesis and the roles of T3SS effectors in this process.
The canonical phagosome
Phagocytosis, or the internalization of particles by professional phagocytes such as macrophages and dendritic cells, is a critical host defense step during infection with bacteria. Depending on the cell type, a cascade of degradative anti-bacterial processes are initiated by the initial binding and engulfment of pathogens, including the production of superoxide and nitric oxide radicals. Concurrently the newly formed phagosome rapidly acidifies, due to the activity of the vacuolar ATPase (vATPase), a large macromolecular complex that is recruited to the phagosomal membrane. Fusion with lysosomes plays a critical role in this process and results in the delivery of lysosomal hydrolases, or endosomal proteases, that are active in the acidified (pH <5) lumen of the phagosome. Supplemented further by the activities of cationic proteins and antimicrobial peptides such as the defensins the mature phagosome is extremely bactericidal and in some cell types also functions as the source of antigenic peptides that are required for the development of an adaptive immune response against individual pathogens.
To survive inside the host cell intracellular bacterial pathogens have adopted a variety of mechanisms to evade intra-phagosomal killing and establish a replicative intracellular niche (for recent review [1]). Perhaps the most obvious approach to this problem is to lyse the phagosomal membrane and escape into the cytoplasm of the host cell, as exemplified by Listeria spp and Shigella spp. However, other bacteria, including Salmonella, have developed alternative ways to evade the arsenal of antimicrobial activities while still remaining within a membrane-bound vacuole or modified phagosome.
Because phagosome biogenesis is determined by its content, in addition to other factors including cell type (reviewed in [2]), there is no de facto default pathway; although latex bead-containing phagosomes have been used as a useful standard with which pathogen-containing phagosomes can be compared. Biogenesis of these phagosomes is a highly synchronous process typified by the sequential acquisition of specific cellular proteins delivered by fusion, either transient or complete, with different endosomal compartments ultimately resulting in the formation of a phagolysosome [3]. Thus early phagosomes (within 30 min following formation) transiently acquire a cohort of proteins typically restricted to early or sorting endosomes, including EEA1, Rab5 and Transferrin receptor. These proteins are then replaced by components of late endosomes and lysosomes, such as vATPase, lysosome-associated membrane proteins (Lamps; also known as LIMPs or lgps) and the lysosomal hydrolases such as the cathepsins. Although this is undoubtedly an oversimplified model, it is a useful benchmark with which pathogen-containing phagosomes can be compared.
The Salmonella-Containing Vacuole
Early studies of SCV biogenesis, which were carried out several years before proteomic analysis of LBP, found that while Lamps were enriched on SCV membranes, other endolysosomal marker proteins were not detected. In particular, neither the mannose 6-phosphate receptors nor lysosomal hydrolases could be detected at significant levels [4–6]. Based on these findings a model was developed based on the idea that Salmonella regulates SCV biogenesis by limiting interactions with the endocytic pathway and particularly by blocking SCV-lysosome fusion. This hypothesis rapidly gained acceptance and subsequent studies concentrated largely on identifying the bacterial factors involved in blocking lysosome fusion with the SCV. Recently, however, advances in live cell imaging technology together with an improved understanding of eukaryotic membrane trafficking events are providing new insights into SCV biogenesis.
For the sake of simplification SCV biogenesis can be separated into 3 stages; early (<30 min p.i.), intermediate (30 min –approx 5 h p.i.) and the late (> 5h p.i.), each of which is associated with specific sets or subsets of T3SS effectors (Fig 1 and Table 1). By far the best-documented aspect of SCV biogenesis is the sequential delivery of endolysosomal membrane proteins that defines the conversion of early to intermediate SCVs. Thus the early SCV membrane is highly enriched in early endosome membrane markers, including EEA1, Rab5 and transferrin receptor, which are replaced within 20–40 min with late endosomal/lysosomal markers including Lamps and vATPase [7,8]. This change in membrane content is accompanied by a decrease in the luminal pH (pHscv) to <4.5 [9,10] and redistribution of the SCV to a predominantly juxtanuclear position near the microtubule organizing center (MTOC) [11]. While this seems to hold true in most cell types studied, in phagocytic cells the mechanism of uptake, i.e. invasion vs phagocytosis, may affect SCV biogenesis especially with respect to acquisition of lysosomal proteins and acidification [9,12]. These data alone do not suggest any significant deviation from “canonical” phagosome biogenesis, particularly since factors such as particle size or surface charge can influence this process [13]. However, neither cathepsins nor the MPRs, which are responsible for trafficking cathepsins from the biosynthetic pathway to the endolysosomal pathway, were enriched in the SCV [4–6] suggesting a novel maturation pathway [4]. Conflicting with this model other studies have since suggested that SCVs can and do fuse with terminal lysosomes and maintain dynamic interactions with the endocytic pathway over many hours [14,15]. So how can these apparently mutually exclusive findings be resolved? One possibility is that Salmonella affects MPR trafficking/recycling rather than lysosome-SCV fusion. Indeed, at steady state MPR is primarily found in the TGN, since it is rapidly recycled following delivery of bound ligands (such as cathepsins) to the endolysosomal pathway. If recycling to the TGN is delayed the receptor is rapidly degraded and delivery of ligand to lysosomes is abrogated [16]. This would explain both low levels of MPR and cathepsins in the SCV. Alternatively, fusion with lysosomes, if not completely blocked may be delayed as indicated by several studies comparing delivery of lysosomal hydrolases, or content, to SCVs containing live wild type Salmonella with those containing non-replicative mutants or other similar particles [6,14,15]. A third possibility is that Salmonella have developed an alternative mechanism to reduce the bactericidal activity of the phagolysosomal environment, which could explain delayed maturation of cathepsins in SCVs [17,18].
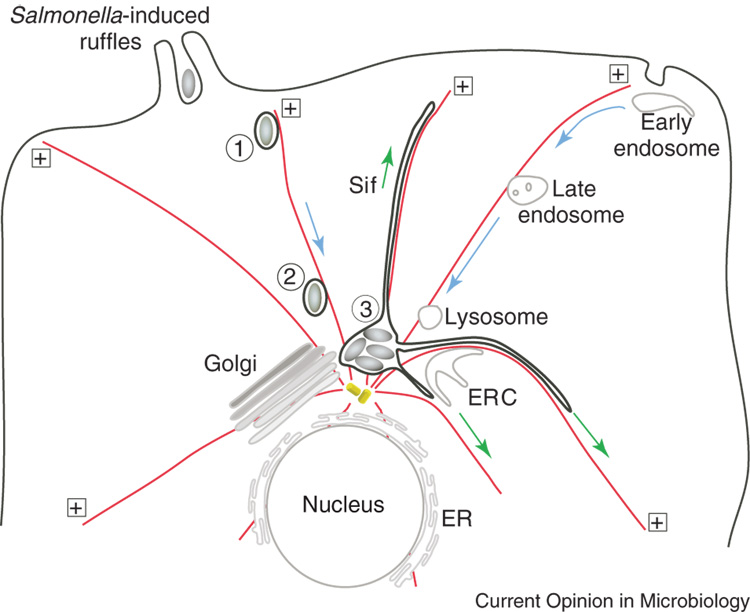
Figure 1.
Schematic representation of SCV biogenesis. Salmonella invade non phagocytic cells by inducing membrane ruffles on the plasma membrane. The bacteria are internalized into the early SCV (1), which is characterized by the presence of markers of the early endocytic pathway such as EEA1 and rab5. Within 15–60 min early endosome proteins are replaced by proteins normally found on late endosome or lysosomes, such as Lamps and vATPase, and SCVs are predominantly localized near the nucleus (2). Finally after 4–6 hr bacterial replication is initiated and Sif tubules extend radially along microtubules (3). In non-polarized epithelial cells microtubules emanate from the juxtanuclear MTOC forming a radial array of uniformly polarized microtubules whose plus ends are closest to the plasma membrane. In these cells the Golgi, lysosomes and endoplasmic recycling compartment (ERC) are concentrated near the MTOC. The localization of these compartments is mediated largely by interactions with microtubule-based motors such as dynein and kinesin, which mediate retrograde (blue arrows) and anterograde movement (green arrows) respectively. Salmonella utilize the same cellular system for translocation of SCVs towards the nucleus and subsequent extension of Sifs.
Table 1.
Stages of SCV biogenesis
| SCV stage | Characteristics | Host cell markers | T3SS effectors implicated | References |
|---|---|---|---|---|
| Early (<30 min) | Simple vacuole or spacious phagosome. | EEA1, rab5a, rab5b, rab5c, transferrin receptor | T3SS1: SipA, SopA, SopB, SpiC/SsaB T3SS2: SpiC | [7,27,38,57] |
| Intermediate (30 min – 5 h) | Vacuole primarily in juxtanuclear position | Lamps, vATPase, rab7, rab11a, rab11b | T3SS1: SopB, SipA T3SS2: SSeF, SseG, SpiC/SsaB, SteC, SseJ/SifC | [7,27,57] |
| Late (>5 h) | Initiation of intracellular replication and formation of tubules (Sifs) radiating throughout cells. Microtubule and actin accumulation around juxtanuclear SCV. | Lamps, vATPase, rab7, rab9 | T3SS2 PipB2, SifA, SopD2, SpiC/SsaB, SseF, SseG, SseJ/SifC, SteC, | [20,27,42,48,53,57] |
Branching Out – Salmonella induced tubules radiate from the SCV into the host cell cytoplasm
The third stage of SCV biogenesis is characterized by bacterial replication and the centrifugal elongation of tubules from the surface of the SCV both of which are initiated within approximately 4–6 hr. Originally, named Sifs, for Salmonella-induced filaments, these tubules have been best characterized in epithelial cells although they can form in other cell types [19]. Sifs have not yet been shown to form in vivo but in cultured epithelial cells they form a dramatic phenotype that distinguishes Salmonella from other intra vacuolar pathogens. Sifs were first discovered because they are highly enriched in Lamp, but they also contain vATPase, the late endosomal lipid lysobisphosphatidic acid, cathepsin D and Rab7 [20].
Centrifugal tubular extension of endolysosomal compartments is certainly not unique to Salmonella-infected cells. Similar tubular lysosomes or endosomes have been described in uninfected macrophages, monocytes, fibroblasts and dendritic cells [21–24]. Nevertheless, there is no doubt that Salmonella induces the formation of extensive Lamp enriched tubules and, although the physiological relevance of this phenomenon remains unclear, the phenotype has been useful in dissecting the roles of individual T3SS effector proteins.
Life in the balance – T3SS effector proteins in SCV/Sif biogenesis
While the debate over whether or not Salmonella block SCV-lysosome fusion continues there is no question that T3SS effectors are required to establish the intracellular niche. Nonetheless, the contributions of individual effectors to SCV biogenesis remain obscure, largely because of redundancy in function so that individual deletion mutants usually have little if any phenotype either in vivo or in vitro. Of the two T3SS in Salmonella it is T3SS2 that has been most conclusively shown to control intracellular events; it is also induced intracellularly and translocates effectors into the host cell across the SCV membrane. In contrast, the T3SS1 is induced extracellularly and translocates effectors across the plasma membrane in order to promote invasion of non-phagocytic cells. Following invasion the T3SS1 system is down regulated but can continue to translocate effectors across the SCV membrane for some time. Switching between T3SS1 and T3SS2 is not well understood, but it is now evident that there is overlap between the two systems and several T3SS1 effectors are implicated in SCV biogenesis and intracellular replication (Table 2).
Table 2.
List of T3SS effector proteins that have been implicated in SCV biogenesis.
| Effector | Intracellular localization | Enzymatic activity | Host target | Function with respect to SCV | References | |
|---|---|---|---|---|---|---|
| Translocated by Salmonella | Ectopically expressed | |||||
| SipA/SspA (T3SS1) | “Foci” in the vicinity of bacteria at early times (≤ 30 min p.i.), SCV at later times (≤ 8 h p.i.) | Cortical actin | Actin | SCV positioning | [27,58–60] | |
| SopA (T3SS1) | Mitochondria | Mitochondria | E3 ubiquitin ligase | HsRMA1 | SCV integrity | [25,61,62] |
| SopB/SigD (T3SS1) | SCV | Membranes of swollen vesicles, lamellipodia | Inositol polyphosphate phosphatase | Membrane phospholipids | Promotes membrane fission during invasion, PI(3)P production in and VAMP8 recruitment to ruffles, Lamp acquisition to SCV | [28,29,32,33,63–65] |
| SopD (T3SS1/T3SS2) | Cytosol | Cytosol and vesicles | Membrane fission and macropinosome formation during invasion | [35,36,66] | ||
| PipB (T3SS2) | SCV, Sifs | [67] | ||||
| PipB2 (T3SS2) | SCV, Sifs | Vesicles at cell periphery | Kinesin-1 | Anterograde movement of Sifs and late endosomes/lysosomes | [19,48,49] | |
| SifA (T3SS2) | SCV, Sifs | Lamp-enriched tubules and vesicles, plasma membrane, cytosol, nucleus | SKIP, Rab7 | SCV positioning, membrane integrity and dynamics, Sif formation | [45,47,68–73] | |
| SifB (T3SS2) | SCV, Sifs | Cytosol | [73] | |||
| SopD2 (T3SS2) | SCV, Sifs | Lamp-rich vesicles and tubules | Sif formation | [35,66] | ||
| SpiC/SsaB (T3SS2) | Conflicting data | Cytosol and nucleus | Hook 3, TassC | Inhibition of vesicle fusion with SCV, VAP formation, Sif formation | [37–39,74–77] | |
| SseF (T3SS2) | SCV, Sifs | Microtubule bundles, Golgi network | SCV positioning and motility, Sif formation, microtubule bundling | [43–45,77–79] | ||
| SseG (T3SS2) | SCV, Sifs | Golgi network, perinuclear aggregates, microtubule bundles | SCV positioning, and motility, Sif formation, microtubule bundling | [11,43–45,77–79] | ||
| SseI/SrfH | VAP | Membrane ruffles and focal adhesions | Filamin, TRIP6 | Actin rearrangements | [55,80,81] | |
| SseJ/SifC (T3SS2) | SCV, Sifs | “Globular membranous compartments” | Deacylase | SCV membrane dynamics, negative regulation of Sifs | [50,51,73] | |
| SspH2 (T3SS2) | VAP | Sites of actin polymerization, membrane ruffles and perinuclear region | Inhibits actin polymerization in vitro | Filamin, profilin | [55] | |
| SteC (STM1698) (T3SS2) | SCV, Sifs, VAP | Cytosolic | Kinase | Actin cytoskeleton | Required for VAP | [54] |
SKIP, SifA and kinesin-interacting protein; VAP, vacuole-associated actin polymerization.
T3SS1 effectors mediate early SCV biogenesis
At this time the four T3SS1 effectors that have been directly implicated in SCV biogenesis are; SipA/SspA, SopA, SopB/SigD and SopD. Following invasion SopA is translocated across SCV membrane into the cytosol where it can be ubiquitinated and rapidly degraded, and is associated with an increase in escape from the SCV, although the significance of this remains unclear [25].
In contrast, SopB and SipA can be detected in cells for many hours after infection [26,27]. SopB is a phosphoinositide (PI) phosphatase that contributes to efficient closing off of the phagocytic cup [28] by hydrolysis of PI(4,5)P2 and has also been shown to be required for PI3P accumulation on the newly formed SCV [29]. The role of PI3P in SCV biogenesis remains unclear; it is required for EEA1, Vamp8 and Lamp1, but not rab5, recruitment [29–33]. It may well be critical for SCV biogenesis but this has not been conclusively demonstrated and furthermore the in vivo substrate, or substrates, of SopB phosphatase activity remain undefined. Nonetheless, since PIs are involved either directly or indirectly in all eukaryotic cell processes, it is likely that SopB activity in the SCV membrane has additional pleiotropic effects [34]. Another T3SS1 effector that is expressed following invasion is SopD [35], a protein that may act cooperatively with SopB at least during initial formation of the phagosome [36]. Lastly, SipA is an actin binding protein that has recently been shown to induce late endosome redistribution in infected cells and also to cooperate with the T3SS2 effector SifA to maintain SCV positioning at late time points [27].
T3SS2 effectors mediate intermediate and late SCV biogenesis
In comparison to the aforementioned T3SS1 effectors T3SS2 effectors seem to mediate generally later steps in SCV biogenesis, especially; movement of the SCV to the juxtanuclear region and the subsequent maintenance of this position, formation of an actin meshwork around the SCV and anterograde extension of Sifs along microtubules. The only T3SS2 effector that has been implicated in early SCV biogenesis is SpiC/SsaB, a protein that was shown to inhibit membrane fusion [37,38]. But other data suggests that SpiC is a component of the T3SS2 translocon rather than an effector [39] and this issue remains unresolved. Redistribution of SCVs from the cell periphery to the MTOC/juxtanuclear region is mediated at least in part by the small GTP-binding protein rab7 together with its effector RILP, which are required for recruitment of the microtubule-based motor dynein [40–42]. Once at the MTOC two T3SS2 effectors that have the ability to interact with one another, SseG and SseF, are required to maintain SCV positioning over longer periods of time [43–45]. SseF and SseG, together with SifA, have also been shown to be involved in redirecting exocytic cargo vesicles to the SCV [46]. SifA is essential for Sif formation, since mutants lacking SifA do not form Sifs and eventually escape from the SCV. Although the molecular basis of SifA activity remains unclear at least one of its functions may be to down-regulate the recruitment the microtubule-based motor kinesin on the SCV via interactions with a host protein SKIP [47]. Certainly, at some point following dynein-dependent movement of the SCV to the MTOC, the emphasis switches to kinesin-dependent anterograde extension of Sif tubules away from the SCV/MTOC. The T3SS2 effector PipB2 enhances this process likely via direct interaction with kinesin light chain [48,49]. Sif formation involves at least one other T3SS2 effector, the deacylase SseJ, that seems to act as a counter balance to SifA since it down-regulates Sif tubule formation and, unlike the sifA deletion mutant, the double sifA sseJ deletion mutant does not escape from the SCV [50–52].
The third phenotype associated with T3SS2 effectors is the formation of an F-actin meshwork in the vicinity of the SCV, also known as vacuole-associated actin polymerizations (VAP), a process that is associated with SCV membrane integrity [53]. Three T3SS2 effectors, namely SteC, SseI and SspH2, have been implicated in this process although their roles remain obtuse. SteC has homology to the human kinase Raf-1 and its kinase activity is required for actin remodelling although not for SCV membrane integrity [54]. SseI and SspH2 can both interact with the actin cross-linking protein filamin yet neither of these effectors is essential for VAP formation, which instead requires another virulence factor SpvB that is encoded on the Salmonella virulence plasmid [55]. Interestingly, SspH2 co-localizes with VAP in infected cells and in vitro can interact with the actin binding protein profilin and decrease the rate of actin polymerization [55].
Conclusions
In spite of a spate of recent papers on the roles of T3SS2 effectors in SCV biogenesis we still know almost nothing about the molecular mechanisms involved. The roles of some effectors such as SseF and SseG are starting to unfold but at the same time two central paradigms are changing. Firstly, it is apparent that T3SS1 effectors are not only involved in invasion and at least some of them have potentially profound roles in SCV biogenesis. Secondly, live cell imaging studies are starting to reveal the dazzling dynamics of SCV and Sif biogenesis and have already shown that these organelles are much more accessible to the endolysosomal pathway than previously believed. So where is the future in SCV research? Almost all of what we know about the mechanisms involved in Salmonella-host cell interactions has come from studies using cultured mammalian cells, primarily non-polarized epithelial cells. Macrophages undoubtedly play an important role in Salmonellosis but it is likely that the outcome for the bacteria is determined by a number of factors such as activation state, mechanism of uptake or type of macrophage. For example, the intestinal macrophages encountered by Typhimurium in human gastroenteritis are likely to bear little resemblance to the circulating macrophages encountered in the mouse model of systemic Salmonellosis. Some macrophages may provide a replicative niche for Salmonella, whereas others do not. One well-documented difference between macrophages and epithelial cells is that Salmonella can induce rapid cell death in macrophages, via caspase 1, whereas in epithelial cells a prosurvival pathway is activated [34]. In addition to macrophages, Salmonella interact with a variety of highly differentiated cell types in vivo each of which may require specific adaptions by the bacteria. Although some studies have partially addressed these concerns by using polarized epithelial cells, cultured dendritic cells or activated macrophages, these one dimensional monocultures used in the lab still bear little resemblance to the highly 3 dimensional in vivo environment. It will be extremely interesting to see if SCV biogenesis in vivo is similar to that seen in vitro.
It is also important to consider the different disease outcomes when Salmonella serovars infect different hosts. For example, oral inoculation with serovar Typhimurium causes a localized gastroenteritis or enterocolitis in higher primates, rabbits and young cattle but not in mice unless they are pretreated with streptomycin in which case a limited colonitis develops [56]. Since individual T3SS effector proteins may have roles only in certain cell types or host environments complete dissection of their roles, as well as resolution of the role of lysosome fusion, in SCV biogenesis may ultimately depend on the development of more sophisticated in vitro models or the analysis of appropriate in vivo infections.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
Many thanks to Leigh Knodler for her insightful comments on the manuscript and for collating the data for Table 2. Work in the author’s laboratory is supported by the Intramural Research Program of the National Institutes of Allergy and Infectious Disease, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Haas A. The phagosome: compartment with a license to kill. Traffic. 2007;8:311–330. doi: 10.1111/j.1600-0854.2006.00531.x. [DOI] [PubMed] [Google Scholar]
- 2.Blander JM, Medzhitov R. On regulation of phagosome maturation and antigen presentation. Nat Immunol. 2006;7:1029–1035. doi: 10.1038/ni1006-1029. [DOI] [PubMed] [Google Scholar]
- 3.Garin J, Diez R, Kieffer S, Dermine JF, Duclos S, Gagnon E, Sadoul R, Rondeau C, Desjardins M. The phagosome proteome: insight into phagosome functions. J Cell Biol. 2001;152:165–180. doi: 10.1083/jcb.152.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia-del Portillo F, Finlay BB. Targeting of Salmonella typhimurium to vesicles containing lysosomal membrane glycoproteins bypasses compartments with mannose 6-phosphate receptors. J Cell Biol. 1995;129:81–97. doi: 10.1083/jcb.129.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rathman M, Barker LP, Falkow S. The unique trafficking pattern of Salmonella typhimurium-containing phagosomes in murine macrophages is independent of the mechanism of bacterial entry. Infect Immun. 1997;65:1475–1485. doi: 10.1128/iai.65.4.1475-1485.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garvis SG, Beuzon CR, Holden DW. A role for the PhoP/Q regulon in inhibition of fusion between lysosomes and Salmonella-containing vacuoles in macrophages. Cell Microbiol. 2001;3:731–744. doi: 10.1046/j.1462-5822.2001.00153.x. [DOI] [PubMed] [Google Scholar]
- 7.Steele-Mortimer O, Meresse S, Gorvel JP, Toh BH, Finlay BB. Biogenesis of Salmonella typhimurium-containing vacuoles in epithelial cells involves interactions with the early endocytic pathway. Cell Microbiol. 1999;1:33–49. doi: 10.1046/j.1462-5822.1999.00003.x. [DOI] [PubMed] [Google Scholar]
- 8.Smith AC, Cirulis JT, Casanova JE, Scidmore MA, Brumell JH. Interaction of the Salmonella-containing vacuole with the endocytic recycling system. J Biol Chem. 2005;280:24634–24641. doi: 10.1074/jbc.M500358200. [DOI] [PubMed] [Google Scholar]
- 9.Drecktrah D, Knodler LA, Ireland R, Steele-Mortimer O. The mechanism of Salmonella entry determines the vacuolar environment and intracellular gene expression. Traffic. 2006;7:39–51. doi: 10.1111/j.1600-0854.2005.00360.x. [DOI] [PubMed] [Google Scholar]
- 10.Rathman M, Sjaastad MD, Falkow S. Acidification of phagosomes containing Salmonella typhimurium in murine macrophages. Infect Immun. 1996;64:2765–2773. doi: 10.1128/iai.64.7.2765-2773.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salcedo SP, Holden DW. SseG, a virulence protein that targets Salmonella to the Golgi network. EMBO J. 2003;22:5003–5014. doi: 10.1093/emboj/cdg517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tobar JA, Gonzalez PA, Kalergis AM. Salmonella escape from antigen presentation can be overcome by targeting bacteria to Fc gamma receptors on dendritic cells. J Immunol. 2004;173:4058–4065. doi: 10.4049/jimmunol.173.6.4058. [DOI] [PubMed] [Google Scholar]
- 13.Oh YK, Swanson JA. Different fates of phagocytosed particles after delivery into macrophage lysosomes. J Cell Biol. 1996;132:585–593. doi: 10.1083/jcb.132.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oh YK, Alpuche-Aranda C, Berthiaume E, Jinks T, Miller SI, Swanson JA. Rapid and complete fusion of macrophage lysosomes with phagosomes containing Salmonella typhimurium. Infect Immun. 1996;64:3877–3883. doi: 10.1128/iai.64.9.3877-3883.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drecktrah D, Knodler LA, Howe D, Steele-Mortimer O. Salmonella trafficking is defined by continuous dynamic interactions with the endolysosomal system. Traffic. 2007;8:212–225. doi: 10.1111/j.1600-0854.2006.00529.x.Using live cell imaging to investigate SCV biogenesis, the authors demonstrate that SCVs fuse directly with terminal lysosomes and also remain accessible to incoming endocytic traffic.
- 16.Arighi CN, Hartnell LM, Aguilar RC, Haft CR, Bonifacino JS. Role of the mammalian retromer in sorting of the cation-independent mannose 6-phosphate receptor. J Cell Biol. 2004;165:123–133. doi: 10.1083/jcb.200312055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hashim S, Mukherjee K, Raje M, Basu SK, Mukhopadhyay A. Live Salmonella modulate expression of Rab proteins to persist in a specialized compartment and escape transport to lysosomes. J Biol Chem. 2000;275:16281–16288. doi: 10.1074/jbc.275.21.16281. [DOI] [PubMed] [Google Scholar]
- 18.Hang HC, Loureiro J, Spooner E, van der Velden AW, Kim YM, Pollington AM, Maehr R, Starnbach MN, Ploegh HL. Mechanism-based probe for the analysis of cathepsin cysteine proteases in living cells. ACS Chem Biol. 2006;1:713–723. doi: 10.1021/cb600431a. [DOI] [PubMed] [Google Scholar]
- 19.Knodler LA, Vallance BA, Hensel M, Jackel D, Finlay BB, Steele-Mortimer O. Salmonella type III effectors PipB and PipB2 are targeted to detergent-resistant microdomains on internal host cell membranes. Mol Microbiol. 2003;49:685–704. doi: 10.1046/j.1365-2958.2003.03598.x. [DOI] [PubMed] [Google Scholar]
- 20.Brumell JH, Tang P, Mills SD, Finlay BB. Characterization of Salmonella-induced filaments (Sifs) reveals a delayed interaction between Salmonella-containing vacuoles and late endocytic compartments. Traffic. 2001;2:643–653. doi: 10.1034/j.1600-0854.2001.20907.x. [DOI] [PubMed] [Google Scholar]
- 21.Nichols BA. Uptake and digestion of horseradish peroxidase in rabbit alveolar macrophages. Formation of a pathway connecting lysosomes to the cell surface. Lab Invest. 1982;47:235–246. [PubMed] [Google Scholar]
- 22.Swanson JA, Locke A, Ansel P, Hollenbeck PJ. Radial movement of lysosomes along microtubules in permeabilized macrophages. J Cell Sci. 1992;103(Pt 1):201–209. doi: 10.1242/jcs.103.1.201. [DOI] [PubMed] [Google Scholar]
- 23.Vyas JM, Kim YM, Artavanis-Tsakonas K, Love JC, Van der Veen AG, Ploegh HL. Tubulation of class II MHC compartments is microtubule dependent and involves multiple endolysosomal membrane proteins in primary dendritic cells. J Immunol. 2007;178:7199–7210. doi: 10.4049/jimmunol.178.11.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buckley IK. The lysosomes of cultured chick embryo cells. A correlated light and electron microscopic study. Lab Invest. 1973;29:411–421. [PubMed] [Google Scholar]
- 25.Zhang Y, Higashide W, Dai S, Sherman DM, Zhou D. Recognition and ubiquitination of Salmonella type III effector SopA by a ubiquitin E3 ligase, HsRMA1. J Biol Chem. 2005;280:38682–38688. doi: 10.1074/jbc.M506309200. [DOI] [PubMed] [Google Scholar]
- 26.Drecktrah D, Knodler LA, Galbraith K, Steele-Mortimer O. The Salmonella SPI1 effector SopB stimulates nitric oxide production long after invasion. Cell Microbiol. 2005;7:105–113. doi: 10.1111/j.1462-5822.2004.00436.x. [DOI] [PubMed] [Google Scholar]
- 27.Brawn LC, Hayward RD, Koronakis V. Salmonella SPI1 Effector SipA Persists after Entry and Cooperates with a SPI2 Effector to Regulate Phagosome Maturation and Intracellular Replication. Cell Host & Microbe. 2007;1:63–75. doi: 10.1016/j.chom.2007.02.001.This study shows that a T3SS1 effector can have a defined role in SCV biogenesis and can temporally and functionally overlap with T3SS2 activity.
- 28.Terebiznik MR, Vieira OV, Marcus SL, Slade A, Yip CM, Trimble WS, Meyer T, Finlay BB, Grinstein S. Elimination of host cell PtdIns(4,5)P(2) by bacterial SigD promotes membrane fission during invasion by Salmonella. Nat Cell Biol. 2002;4:766–773. doi: 10.1038/ncb854. [DOI] [PubMed] [Google Scholar]
- 29.Dukes JD, Lee H, Hagen R, Reaves BJ, Layton AN, Galyov EE, Whitley P. The secreted Salmonella dublin phosphoinositide phosphatase, SopB, localizes to PtdIns(3)P-containing endosomes and perturbs normal endosome to lysosome trafficking. Biochem J. 2006;395:239–247. doi: 10.1042/BJ20051451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pattni K, Jepson M, Stenmark H, Banting G. A PtdIns(3)P-specific probe cycles on and off host cell membranes during Salmonella invasion of mammalian cells. Curr Biol. 2001;11:1636–1642. doi: 10.1016/s0960-9822(01)00486-9. [DOI] [PubMed] [Google Scholar]
- 31.Scott CC, Cuellar-Mata P, Matsuo T, Davidson HW, Grinstein S. Role of 3-phosphoinositides in the maturation of Salmonella-containing vacuoles within host cells. J Biol Chem. 2002;277:12770–12776. doi: 10.1074/jbc.M110399200. [DOI] [PubMed] [Google Scholar]
- 32.Hernandez LD, Hueffer K, Wenk MR, Galan JE. Salmonella modulates vesicular traffic by altering phosphoinositide metabolism. Science. 2004;304:1805–1807. doi: 10.1126/science.1098188. [DOI] [PubMed] [Google Scholar]
- 33.Dai S, Zhang Y, Weimbs T, Yaffe MB, Zhou D. Bacteria-generated PtdIns(3)P Recruits VAMP8 to Facilitate Phagocytosis. Traffic. 2007;8:1365–1374. doi: 10.1111/j.1600-0854.2007.00613.x. [DOI] [PubMed] [Google Scholar]
- 34.Knodler LA, Finlay BB, Steele-Mortimer O. The Salmonella effector protein SopB protects epithelial cells from apoptosis by sustained activation of Akt. J Biol Chem. 2005;280:9058–9064. doi: 10.1074/jbc.M412588200. [DOI] [PubMed] [Google Scholar]
- 35.Brumell JH, Kujat-Choy S, Brown NF, Vallance BA, Knodler LA, Finlay BB. SopD2 is a novel type III secreted effector of Salmonella typhimurium that targets late endocytic compartments upon delivery into host cells. Traffic. 2003;4:36–48. doi: 10.1034/j.1600-0854.2003.40106.x. [DOI] [PubMed] [Google Scholar]
- 36.Bakowski MA, Cirulis JT, Brown NF, Finlay BB, Brumell JH. SopD acts cooperatively with SopB during Salmonella enterica serovar Typhimurium invasion. Cell Microbiol. 2007 doi: 10.1111/j.1462-5822.2007.01000.x. [DOI] [PubMed] [Google Scholar]
- 37.Shotland Y, Kramer H, Groisman EA. The Salmonella SpiC protein targets the mammalian Hook3 protein function to alter cellular trafficking. Mol Microbiol. 2003;49:1565–1576. doi: 10.1046/j.1365-2958.2003.03668.x. [DOI] [PubMed] [Google Scholar]
- 38.Uchiya K, Barbieri MA, Funato K, Shah AH, Stahl PD, Groisman EA. A Salmonella virulence protein that inhibits cellular trafficking. EMBO J. 1999;18:3924–3933. doi: 10.1093/emboj/18.14.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu XJ, Liu M, Holden DW. SsaM and SpiC interact and regulate secretion of Salmonella pathogenicity island 2 type III secretion system effectors and translocators. Mol Microbiol. 2004;54:604–619. doi: 10.1111/j.1365-2958.2004.04297.x. [DOI] [PubMed] [Google Scholar]
- 40.Harrison RE, Brumell JH, Khandani A, Bucci C, Scott CC, Jiang X, Finlay BB, Grinstein S. Salmonella impairs RILP recruitment to Rab7 during maturation of invasion vacuoles. Mol Biol Cell. 2004;15:3146–3154. doi: 10.1091/mbc.E04-02-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marsman M, Jordens I, Kuijl C, Janssen L, Neefjes J. Dynein-mediated vesicle transport controls intracellular Salmonella replication. Mol Biol Cell. 2004;15:2954–2964. doi: 10.1091/mbc.E03-08-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guignot J, Caron E, Beuzon C, Bucci C, Kagan J, Roy C, Holden DW. Microtubule motors control membrane dynamics of Salmonella-containing vacuoles. J Cell Sci. 2004;117:1033–1045. doi: 10.1242/jcs.00949. [DOI] [PubMed] [Google Scholar]
- 43.Ramsden AE, Mota LJ, Munter S, Shorte SL, Holden DW. The SPI-2 type III secretion system restricts motility of Salmonella-containing vacuoles. Cell Microbiol. 2007;9:2517–2529. doi: 10.1111/j.1462-5822.2007.00977.x.Live cell imaging was used to show that two T3SS2 effectors, SseG and SseF, prevent dispersion of SCVs from the juxtanuclear region.
- 44.Abrahams GL, Muller P, Hensel M. Functional dissection of SseF, a type III effector protein involved in positioning the salmonella-containing vacuole. Traffic. 2006;7:950–965. doi: 10.1111/j.1600-0854.2006.00454.x. [DOI] [PubMed] [Google Scholar]
- 45.Deiwick J, Salcedo SP, Boucrot E, Gilliland SM, Henry T, Petermann N, Waterman SR, Gorvel JP, Holden DW, Meresse S. The translocated Salmonella effector proteins SseF and SseG interact and are required to establish an intracellular replication niche. Infect Immun. 2006;74:6965–6972. doi: 10.1128/IAI.00648-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuhle V, Abrahams GL, Hensel M. Intracellular Salmonella enterica redirect exocytic transport processes in a Salmonella pathogenicity island 2-dependent manner. Traffic. 2006;7:716–730. doi: 10.1111/j.1600-0854.2006.00422.x. [DOI] [PubMed] [Google Scholar]
- 47.Boucrot E, Henry T, Borg JP, Gorvel JP, Meresse S. The intracellular fate of Salmonella depends on the recruitment of kinesin. Science. 2005;308:1174–1178. doi: 10.1126/science.1110225.Using a 2-hybrid screen the authors identified a ubiquitously expressed mammalian protein of unknown function that is targeted by the T3SS2 effector SifA. Depletion of SKIP by RNA interference resulted in decreased stability of SCVs and release of bacteria into the cytosol, effectively the same phenotype as a SifA deletion mutant. SKIP is proposed to down-regulate the recruitment of kinesin to the SCV.
- 48.Knodler LA, Steele-Mortimer O. The Salmonella effector PipB2 affects late endosome/lysosome distribution to mediate Sif extension. Mol Biol Cell. 2005;16:4108–4123. doi: 10.1091/mbc.E05-04-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Henry T, Couillault C, Rockenfeller P, Boucrot E, Dumont A, Schroeder N, Hermant A, Knodler LA, Lecine P, Steele-Mortimer O, et al. The Salmonella effector protein PipB2 is a linker for kinesin-1. Proc Natl Acad Sci U S A. 2006;103:13497–13502. doi: 10.1073/pnas.0605443103.The authors show that PipB2 is required for recruitment of kinesin to the surface of the SCV by interacting directly with the kinesin light cahin.
- 50.Birmingham CL, Jiang X, Ohlson MB, Miller SI, Brumell JH. Salmonella-induced filament formation is a dynamic phenotype induced by rapidly replicating Salmonella enterica serovar typhimurium in epithelial cells. Infect Immun. 2005;73:1204–1208. doi: 10.1128/IAI.73.2.1204-1208.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruiz-Albert J, Yu XJ, Beuzon CR, Blakey AN, Galyov EE, Holden DW. Complementary activities of SseJ and SifA regulate dynamics of the Salmonella typhimurium vacuolar membrane. Mol Microbiol. 2002;44:645–661. doi: 10.1046/j.1365-2958.2002.02912.x. [DOI] [PubMed] [Google Scholar]
- 52.Ohlson MB, Fluhr K, Birmingham CL, Brumell JH, Miller SI. SseJ deacylase activity by Salmonella enterica serovar Typhimurium promotes virulence in mice. Infect Immun. 2005;73:6249–6259. doi: 10.1128/IAI.73.10.6249-6259.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meresse S, Unsworth KE, Habermann A, Griffiths G, Fang F, Martinez-Lorenzo MJ, Waterman SR, Gorvel JP, Holden DW. Remodelling of the actin cytoskeleton is essential for replication of intravacuolar Salmonella. Cell Microbiol. 2001;3:567–577. doi: 10.1046/j.1462-5822.2001.00141.x. [DOI] [PubMed] [Google Scholar]
- 54.Poh J, Odendall C, Spanos A, Boyle C, Liu M, Freemont P, Holden DW. SteC is a Salmonella kinase required for SPI-2-dependent F-actin remodelling. Cell Microbiol. 2007 doi: 10.1111/j.1462-5822.2007.01010.x.In this paper the authors show that a T3SS2 effector has homology with the human kinase Raf-1 and that SteC kinase activity is required for actin remodelling.
- 55.Miao EA, Brittnacher M, Haraga A, Jeng RL, Welch MD, Miller SI. Salmonella effectors translocated across the vacuolar membrane interact with the actin cytoskeleton. Mol Microbiol. 2003;48:401–415. doi: 10.1046/j.1365-2958.2003.t01-1-03456.x. [DOI] [PubMed] [Google Scholar]
- 56.Hapfelmeier S, Ehrbar K, Stecher B, Barthel M, Kremer M, Hardt WD. Role of the Salmonella pathogenicity island 1 effector proteins SipA, SopB, SopE, and SopE2 in Salmonella enterica subspecies 1 serovar Typhimurium colitis in streptomycin-pretreated mice. Infect Immun. 2004;72:795–809. doi: 10.1128/IAI.72.2.795-809.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith AC, Heo WD, Braun V, Jiang X, Macrae C, Casanova JE, Scidmore MA, Grinstein S, Meyer T, Brumell JH. A network of Rab GTPases controls phagosome maturation and is modulated by Salmonella enterica serovar Typhimurium. J Cell Biol. 2007;176:263–268. doi: 10.1083/jcb.200611056.To investigate the role of Rab proteins in SCV biogenesis the authors transfected 48 different GFP- or CFP-rab protein fusions and compared their association with model phagosomes containing trafficking defective Salmonella to SCVs containing wild type Salmonella. They identified 5 Rab proteins that were excluded from SCVs.
- 58.Schlumberger MC, Muller AJ, Ehrbar K, Winnen B, Duss I, Stecher B, Hardt WD. Real-time imaging of type III secretion: Salmonella SipA injection into host cells. Proc Natl Acad Sci U S A. 2005;102:12548–12553. doi: 10.1073/pnas.0503407102.Using time-lapse imaging the authors show delivery of individual effector proteins into host cells in real time.
- 59.Schlumberger MC, Kappeli R, Wetter M, Muller AJ, Misselwitz B, Dilling S, Kremer M, Hardt WD. Two newly identified SipA domains (F1, F2) steer effector protein localization and contribute to Salmonella host cell manipulation. Mol Microbiol. 2007;65:741–760. doi: 10.1111/j.1365-2958.2007.05823.x. [DOI] [PubMed] [Google Scholar]
- 60.McGhie EJ, Hayward RD, Koronakis V. Cooperation between actin-binding proteins of invasive Salmonella: SipA potentiates SipC nucleation and bundling of actin. EMBO J. 2001;20:2131–2139. doi: 10.1093/emboj/20.9.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Layton AN, Brown PJ, Galyov EE. The Salmonella translocated effector SopA is targeted to the mitochondria of infected cells. J Bacteriol. 2005;187:3565–3571. doi: 10.1128/JB.187.10.3565-3571.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Y, Higashide WM, McCormick BA, Chen J, Zhou D. The inflammation-associated Salmonella SopA is a HECT-like E3 ubiquitin ligase. Mol Microbiol. 2006;62:786–793. doi: 10.1111/j.1365-2958.2006.05407.x. [DOI] [PubMed] [Google Scholar]
- 63.Marcus SL, Knodler LA, Finlay BB. Salmonella enterica serovar Typhimurium effector SigD/SopB is membrane-associated and ubiquitinated inside host cells. Cell Microbiol. 2002;4:435–446. doi: 10.1046/j.1462-5822.2002.00202.x. [DOI] [PubMed] [Google Scholar]
- 64.Mason D, Mallo GV, Terebiznik MR, Payrastre B, Finlay BB, Brumell JH, Rameh L, Grinstein S. Alteration of epithelial structure and function associated with PtdIns(4,5)P2 degradation by a bacterial phosphatase. J Gen Physiol. 2007;129:267–283. doi: 10.1085/jgp.200609656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou D, Chen LM, Hernandez L, Shears SB, Galan JE. A Salmonella inositol polyphosphatase acts in conjunction with other bacterial effectors to promote host cell actin cytoskeleton rearrangements and bacterial internalization. Mol Microbiol. 2001;39:248–259. doi: 10.1046/j.1365-2958.2001.02230.x. [DOI] [PubMed] [Google Scholar]
- 66.Jiang X, Rossanese OW, Brown NF, Kujat-Choy S, Galan JE, Finlay BB, Brumell JH. The related effector proteins SopD and SopD2 from Salmonella enterica serovar Typhimurium contribute to virulence during systemic infection of mice. Mol Microbiol. 2004;54:1186–1198. doi: 10.1111/j.1365-2958.2004.04344.x. [DOI] [PubMed] [Google Scholar]
- 67.Knodler LA, Celli J, Hardt WD, Vallance BA, Yip C, Finlay BB. Salmonella effectors within a single pathogenicity island are differentially expressed and translocated by separate type III secretion systems. Mol Microbiol. 2002;43:1089–1103. doi: 10.1046/j.1365-2958.2002.02820.x. [DOI] [PubMed] [Google Scholar]
- 68.Boucrot E, Beuzon CR, Holden DW, Gorvel JP, Meresse S. Salmonella typhimurium SifA effector protein requires its membrane-anchoring C-terminal hexapeptide for its biological function. J Biol Chem. 2003;278:14196–14202. doi: 10.1074/jbc.M207901200. [DOI] [PubMed] [Google Scholar]
- 69.Reinicke AT, Hutchinson JL, Magee AI, Mastroeni P, Trowsdale J, Kelly AP. A Salmonella typhimurium effector protein SifA is modified by host cell prenylation and S-acylation machinery. J Biol Chem. 2005;280:14620–14627. doi: 10.1074/jbc.M500076200. [DOI] [PubMed] [Google Scholar]
- 70.Beuzon CR, Meresse S, Unsworth KE, Ruiz-Albert J, Garvis S, Waterman SR, Ryder TA, Boucrot E, Holden DW. Salmonella maintains the integrity of its intracellular vacuole through the action of SifA. EMBO J. 2000;19:3235–3249. doi: 10.1093/emboj/19.13.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brumell JH, Goosney DL, Finlay BB. SifA, a type III secreted effector of Salmonella typhimurium, directs Salmonella-induced filament (Sif) formation along microtubules. Traffic. 2002;3:407–415. doi: 10.1034/j.1600-0854.2002.30604.x. [DOI] [PubMed] [Google Scholar]
- 72.Brumell JH, Rosenberger CM, Gotto GT, Marcus SL, Finlay BB. SifA permits survival and replication of Salmonella typhimurium in murine macrophages. Cell Microbiol. 2001;3:75–84. doi: 10.1046/j.1462-5822.2001.00087.x. [DOI] [PubMed] [Google Scholar]
- 73.Freeman JA, Ohl ME, Miller SI. The Salmonella enterica serovar typhimurium translocated effectors SseJ and SifB are targeted to the Salmonella-containing vacuole. Infect Immun. 2003;71:418–427. doi: 10.1128/IAI.71.1.418-427.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu XJ, Ruiz-Albert J, Unsworth KE, Garvis S, Liu M, Holden DW. SpiC is required for secretion of Salmonella Pathogenicity Island 2 type III secretion system proteins. Cell Microbiol. 2002;4:531–540. doi: 10.1046/j.1462-5822.2002.00211.x. [DOI] [PubMed] [Google Scholar]
- 75.Abrahams GL, Hensel M. Manipulating cellular transport and immune responses: dynamic interactions between intracellular Salmonella enterica and its host cells. Cell Microbiol. 2006;8:728–737. doi: 10.1111/j.1462-5822.2006.00706.x. [DOI] [PubMed] [Google Scholar]
- 76.Lee AH, Zareei MP, Daefler S. Identification of a NIPSNAP homologue as host cell target for Salmonella virulence protein SpiC. Cell Microbiol. 2002;4:739–750. doi: 10.1046/j.1462-5822.2002.00225.x. [DOI] [PubMed] [Google Scholar]
- 77.Guy RL, Gonias LA, Stein MA. Aggregation of host endosomes by Salmonella requires SPI2 translocation of SseFG and involves SpvR and the fms-aroE intragenic region. Mol Microbiol. 2000;37:1417–1435. doi: 10.1046/j.1365-2958.2000.02092.x. [DOI] [PubMed] [Google Scholar]
- 78.Kuhle V, Jackel D, Hensel M. Effector proteins encoded by Salmonella pathogenicity island 2 interfere with the microtubule cytoskeleton after translocation into host cells. Traffic. 2004;5:356–370. doi: 10.1111/j.1398-9219.2004.00179.x. [DOI] [PubMed] [Google Scholar]
- 79.Kuhle V, Hensel M. SseF and SseG are translocated effectors of the type III secretion system of Salmonella pathogenicity island 2 that modulate aggregation of endosomal compartments. Cell Microbiol. 2002;4:813–824. doi: 10.1046/j.1462-5822.2002.00234.x. [DOI] [PubMed] [Google Scholar]
- 80.Miao EA, Miller SI. A conserved amino acid sequence directing intracellular type III secretion by Salmonella typhimurium. Proc Natl Acad Sci U S A. 2000;97:7539–7544. doi: 10.1073/pnas.97.13.7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Worley MJ, Nieman GS, Geddes K, Heffron F. Salmonella typhimurium disseminates within its host by manipulating the motility of infected cells. Proc Natl Acad Sci U S A. 2006;103:17915–17920. doi: 10.1073/pnas.0604054103. [DOI] [PMC free article] [PubMed] [Google Scholar]