Figure 1. Overall structure of PAE2072.
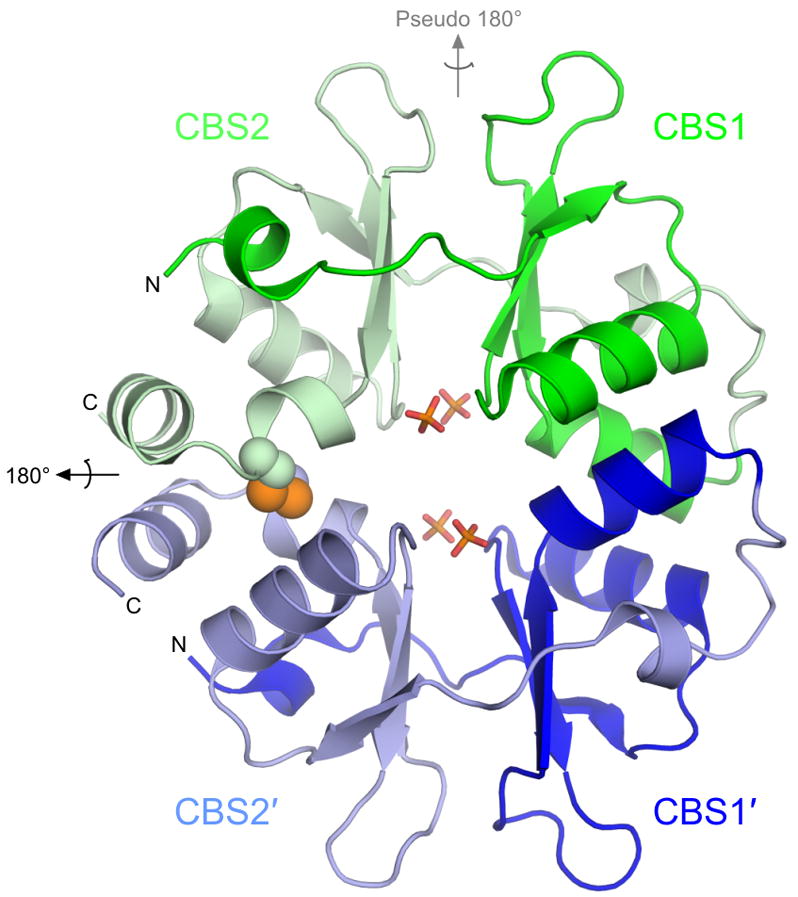
The unliganded PAE2072 dimer is shown in cartoon representation, with each CBS domain colored individually. The intermolecular disulfide bond between cysteines 120 and 120′ is shown as spheres, and the sulfate ions that occupy the same positions as the phosphate groups of AMP in the AMP-bound structure are shown in stick representation. The dimeric two-fold axis is indicated in black, and the pseudo two-fold axis in gray. The core β-α-β-β-α fold conserved in CBS domain proteins is easily identifiable; an additional short helix punctuates the extended N-terminus of each CBS domain.
