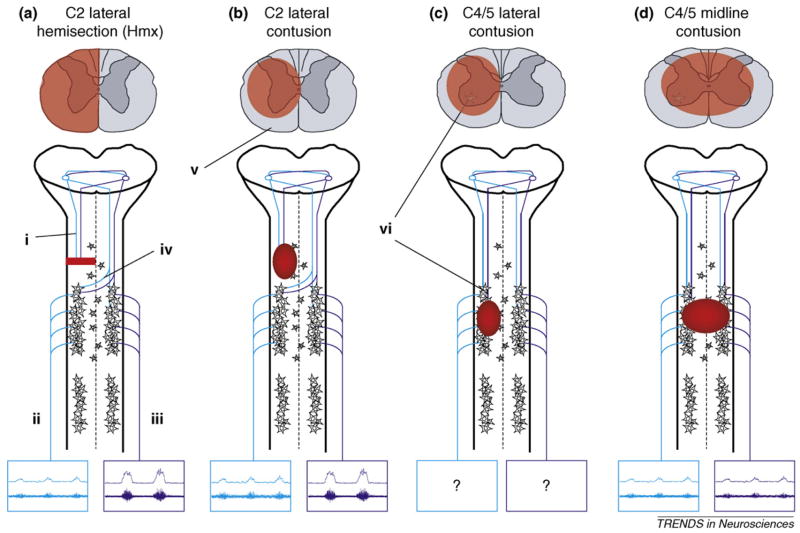
Figure 1.
Schematic diagrams outlining cervical injury models that have been used to examine respiratory function. Diagrams of spinal cord cross-sections demonstrate general morphological features (highlighted in red) of each injury type. Emphasis is given to descending pathways from the medulla (i) to PhMNs, which control diaphragm function via the phrenic nerve. Examples are presented of how each injury type affects efferent burst activity (both raw [top] and integrated [bottom] neurograms) recorded in the phrenic nerves (ii, iii) on each side of the spinal cord. The C2HMx (a) is the most frequently examined model and has provided the best evidence for respiratory neuroplasticity post-SCI. Following interruption of ipsilateral input from the medulla, spontaneous recovery of ipsilateral phrenic nerve activity (ii) has been observed. This recovery, known as the crossed phrenic phenomenon (CPP), is attributed to latent pathways (iv) that cross the spinal midline at the level of PhMNs. Note that following lateral injuries (a,b), in addition to ipsilateral recovery, contralateral phrenic activity is increased relative to normal. As contusive injury occurs more frequently in human SCI, some studies have begun to compare lateral C2 contusions (b) to the C2HMx model. However, depending on the extent of injury, contusive lesions usually result in some degree of white matter sparing (v). Therefore, in addition to the crossed, contralateral pathways of the CPP, some ipsilateral input from the medulla might be spared. As the majority of human SCIs occur in the midcervical region (C4/5), some studies have also begun to address the effects of such injuries on respiration (c, d). How lateral injuries at this level affect phrenic output is yet to be determined. However, as indicated, lesions at the midcervical levels also result in loss of PhMNs (vi). Furthermore, lesions that extend toward the spinal midline (d) are likely to interrupt crossing pathways associated with the CPP.