Figure 2. Expression and constitutive activity of mutant CKLiK proteins.
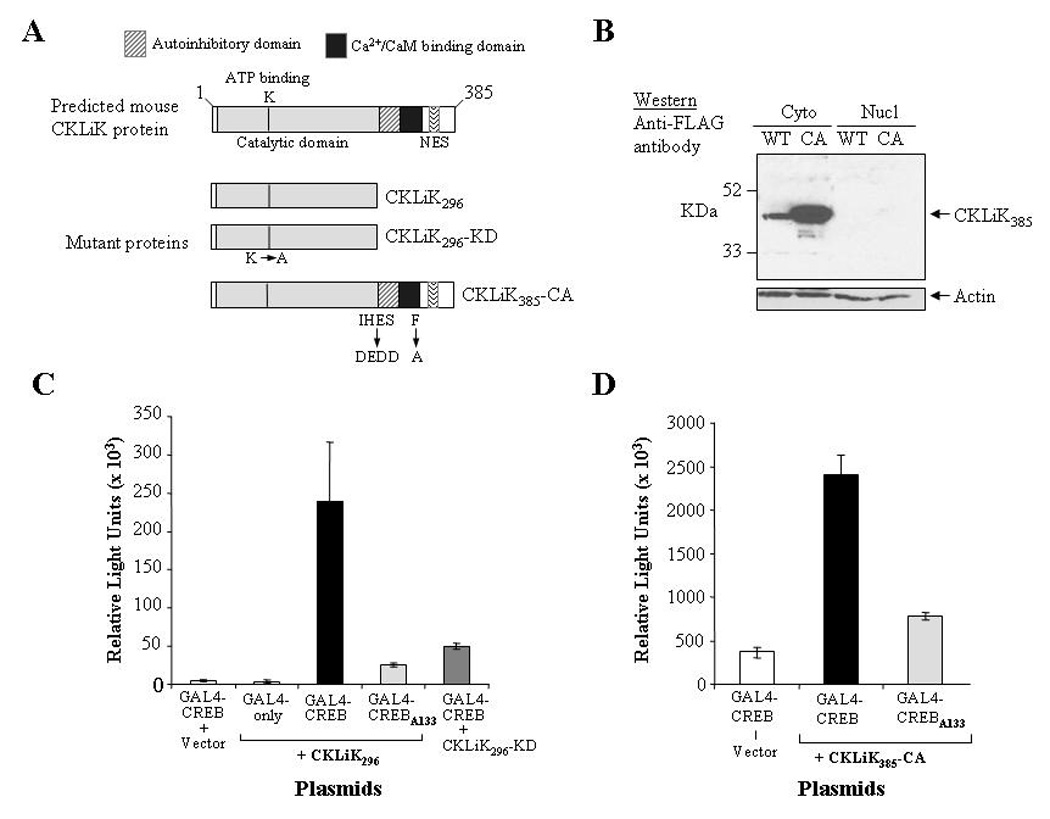
(A) Depicted are the wild-type form of CKLiK and the two constitutively active forms, CKLiK296 (truncated at Gln296) and CKLiK385-CA (internal mutations that mimic Ca2+/CaM binding). Also shown is the “kinase dead” form of the truncated CKLiK (CKLiK296-KD) that contains a mutation in the ATP binding site (Lys52). (B) Results of a Western blot containing cytoplasmic versus nuclear extracts from HEK293 cells transfected with wild-type (WT) or constitutively active (CA) versions of CKLiK demonstrate abundant expression in the cytoplasm, whereas nuclear expression was undetectable. The blot was probed with an anti-FLAG antibody to detect CKLiK expression and with an anti-actin antibody to demonstrate amounts of protein in each lane. (C and D) Activation of CREB by each mutant form of CKLiK was tested by transfecting COS-1 cells with the expression vectors for each mutant kinase together with the two reporter plasmids, pCMV-GAL4-CREBΔb-zip (GAL4-CREB) and p5XGAL4-E1b-luciferase. Control vectors also used were one lacking the CREB activation domain (GAL4-only) and one with a Ser133 to Ala mutation in the CREB motif (GAL4-CREBA133). Levels of luciferase activity were normalized to levels of β-galactosidase expressed from co-transfected plasmids. Values shown are the averages ± SD from triplicate transfections performed in parallel and are representative of at least 3 independent experiments.
