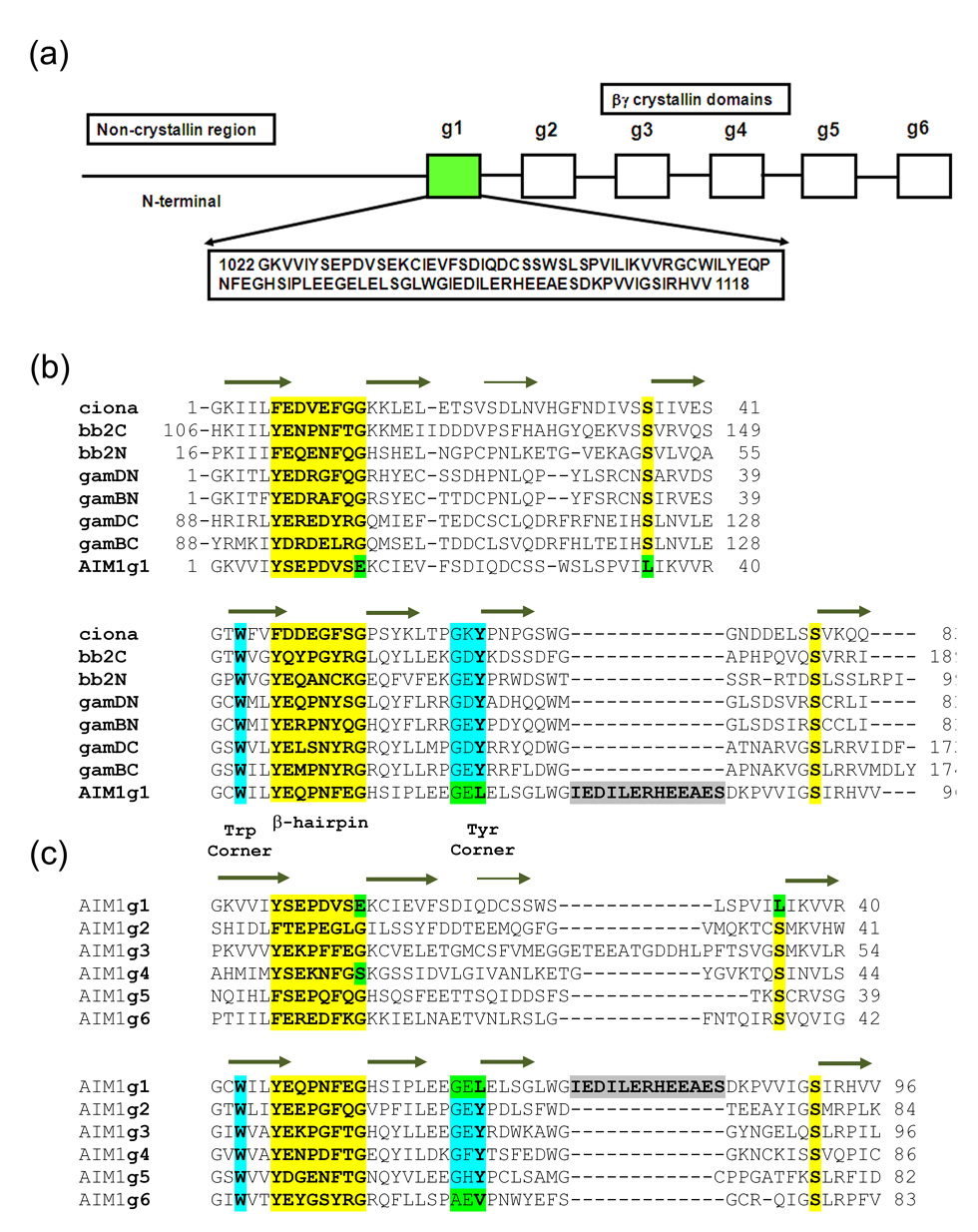
Figure 1.
(a) βγ-Crystallin domain organization in AIM1 with sequence of AIM1g1. (b) Multiple sequence alignment between AIM1g1 and other vertebrate and urochordate lens βγ-crystallins are shown. g1–g6 represents the six crystallin domains of AIM1. (c) Second panel shows alignment of other βγ-crystallin domains of AIM1 protein. An insertion similar to the 10-residue insertion present in motif B of AIM1g1 is present in the A-motif of the AIM1g3. The first and the last domains lack a Tyr-corner. All crucial structural features of the domains are explicitly identified. Mutations at highly invariant residues are marked in green.