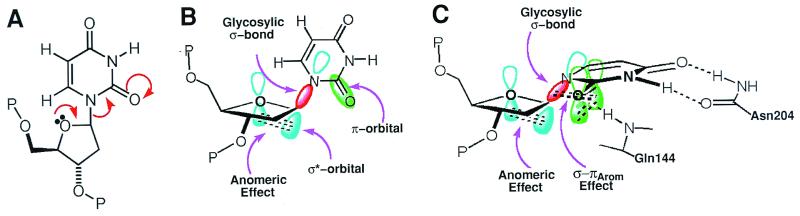
Figure 5.
Structure-based reaction mechanism that resolves the apparent orthogonal paradox for electron transpositions by altering the substrate stereochemistry. (A) A simplified valence-bond representation of the glycosylic bond dissociation hides the paradox that the three electron pairs to be transposed are involved in orthogonal orbitals. (B) In the normal anti-conformation of deoxyuridine, the σ*-orbital involved in the anomeric effect and the π-orbital of the C2⩵O bond are orthogonal to one another, thus preventing orbital overlap. (C) Severe distortions of the deoxyribose and the glycosylic bond in the strained conformation of deoxyuridine enforced by the UDG active center align the pairs of atomic orbitals participating in each electron transposition, thereby electronically coupling the anomeric and σ-πArom effects to promote bond cleavage.