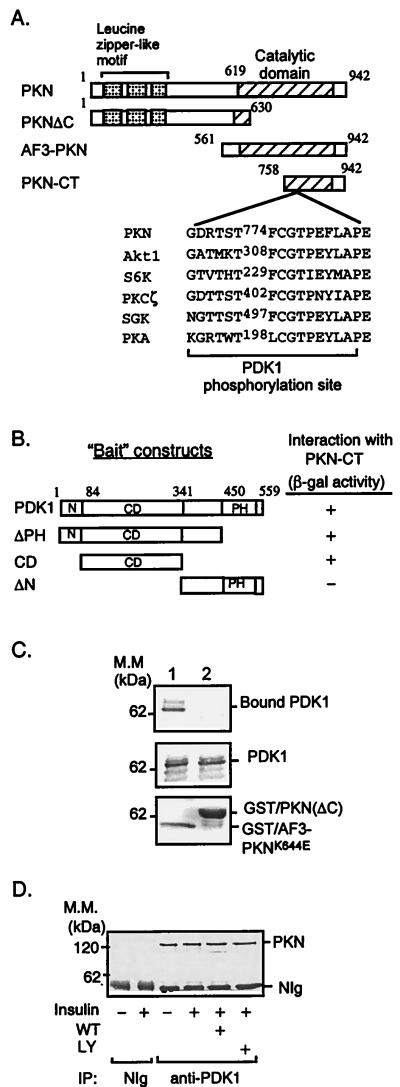
Figure 1.
Interaction of PKN with PDK1. (A) Schematic representation of PKN constructs used to study PKN and PDK1 interaction and phosphorylation. The consensus PDK1 phosphorylation sequences of several PDK1 substrates are shown. (B) Interaction of PDK1 with PKN in the yeast two-hybrid system. cDNAs containing different regions of PDK1 were amplified by PCR and subcloned into the yeast two-hybrid plasmid pGBT9. The interaction between PDK1 fragments and PKN-CT in SFY526 yeast cells was detected by β-galactosidase filter assays as described (14). +, Positive interaction (blue color) was visualized within 30 min; −, no interaction was detected for 24 h. N, Amino terminus; CD, catalytic domain; PH, pleckstrin homology domain. (C) Interaction between PDK1 and PKN in vitro. Lysates of CHO/IR/PDK1 cells (11) were incubated with GST/AF3-PKNK644E (lane 1) or GST/PKN(ΔC) (lane 2) bound to glutathione-Sepharose beads (Sigma). The bound proteins were resolved by SDS/PAGE, transferred onto a nitrocellulose membrane, and examined by immunoblotting with antibody to the hemagglutinin tag (Top). Five percent of cell lysates used in the immunoprecipitation were loaded as a control (Middle). The GST fusion proteins used in the experiments were separated by SDS/PAGE and visualized by Coomassie blue staining (Bottom). Results are representative of three independent experiments. (D) Interaction of PDK1 and PKN in intact cells. CHO/IR cells were serum-starved overnight and treated with or without wortmannin (WT, 200 nM) or LY294002 (LY, 200 μM) for 1 h. Cells then were treated with (+) or without (−) 10 nM insulin for 10 min. Cell lysates were incubated for 8 h at 4°C with affinity-purified polyclonal antibody to PDK1 (11) or NIg bound to protein A beads. PKN coimmunoprecipitated with PDK1 was detected with a mAb to PKN (Transduction Laboratories).