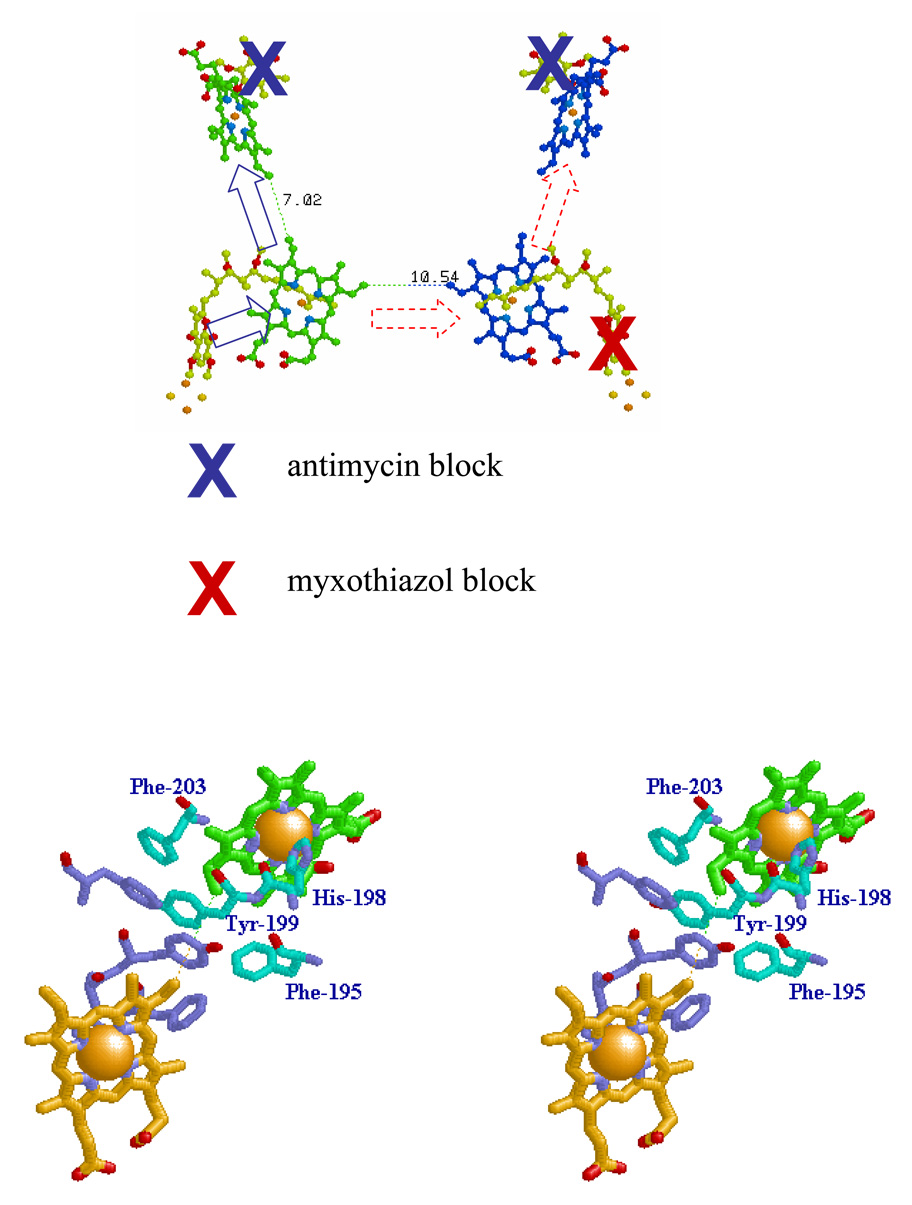
Fig. 3. Schemes to show potential electron transfer pathways involved in electron equilibration between monomers at the level of heme bL.
A. The redox centers of the dimer are shown, and the Qo- and Qi-sites indicated by stigmatellin and antimycin, respectively. The scheme shows the dimer with the Qi-site blocked in both monomers by antimycin (blue crosses), and the Qo-site of one monomer blocked by myxothiazol (red cross). Under these conditions, if electron transfer between hemes bL could occur, both hemes bH could be reduced by turnover of the uninhibited Qo-site (broken red arrows). B. The dimer interface, showing residues referred to in the text. The dotted line shows the 10.21 Å distance between heme bL 4-vinyl groups (taken from PDB file 2qjy, chains A–F).