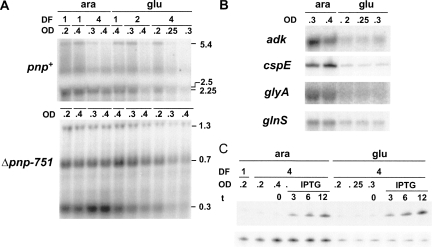
FIGURE 5.
Northern analysis of the pnp and other model transcripts upon S1 depletion. (A,B) Twelve micrograms of total RNA extracted from cultures of C-5698 (pnp+; upper part of panel A and panel B) and C-5707 (Δpnp-751; lower part of panel A), grown as detailed in Materials and Methods, were fractioned by 1.5% denaturating agarose gel electrophoresis, transferred onto a nylon membrane, and hybridized with the radiolabeled riboprobe specific for pnp (A) or the other mRNAs as listed on the left in B. Dilution factor (DF) of each subculture and OD600 at the sampling time are reported on the top of each lane. Length (in kilobases) of pnp transcripts is reported on the right of panel A. Only the gel portions displaying signals are shown. (C) Cultures of C-5699/pGM331 were grown at OD600 = 0.2, diluted 1:4 (DF, dilution factor) in permissive (ara) and nonpermissive (glu) condition, and incubated until OD600 = 0.2 was reached, as described in Materials and Methods. Incubation was carried on for additional 30 min (ara, OD600 = 0.4) or 60 min (glu, OD600 = 0.3) before the addition of 1 mM IPTG.. Samples were taken before (t = 0) and at different time (indicated in minutes) after the induction. Five micrograms of total RNA were fractioned on 6% denaturing polyacrylamide gel, transferred onto a nylon membrane, and hybridized with the tRNAGly-specific radiolabeled oligonucleotide (upper part) or with the cspE-specific riboprobe (lower part).