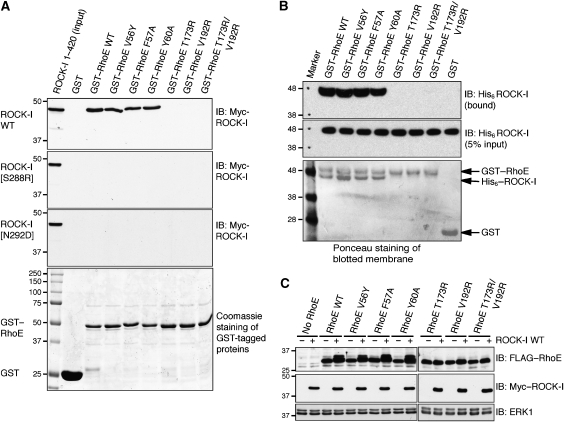
Figure 6.
Disruption of ROCK-I:RhoE interaction and effects on RhoE/ROCK-I responses. (A) The indicated wild-type (WT) and mutant GST–RhoE proteins on glutathione beads were incubated with cell lysates from COS-7 cells transfected with pCAG-myc-ROCK-I1−420 (wild-type and indicated mutants). Proteins that bound to GST–RhoE were resolved by SDS–PAGE and western blotted with anti-myc antibody to identify myc–ROCK-I. The lower panel (Coomassie staining of polyacrylamide gel) shows that equal amounts of each GST–RhoE mutant were used in the binding assay. (B) GST–RhoE was incubated with His-ROCK-I purified from baculovirus-infected insect cells. ROCK-I bound to glutathione agarose immobilised RhoE was identified by western blotting with anti-His antibody. (C) ROCK-I phosphorylation of RhoE in cells. COS-7 cells were co-transfected with the indicated FLAG–RhoE and myc–ROCK-I1−420 expression vectors. After 24 h, cells were lysed and cell lysates were western blotted with anti-FLAG antibody to detect RhoE. ROCK-I-phosphorylated RhoE migrates more slowly. ERK1 panels indicate equivalent loading in each lane.