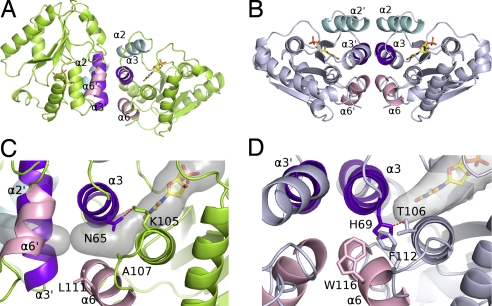
Fig. 2.
Subunit associations of vaccinia virus and human TMP kinases. (A and B) Ribbon representation of the vaccinia virus enzyme in lime green (A) and the human enzyme in pale blue (B) (PDB ID code 1e2d). The interface helices of dimers are in cyan (α2), purple (α3), and pink (α6). (C and D) Close-up of the packing between the helices in vaccinia virus (C) and human (D) enzymes at the dimer interfaces. The α3 helices are packed perpendicularly in the vaccinia enzyme (C) and antiparallel in human TMP kinase (D). His-69 closes the rear of the base binding pocket in the human enzyme and is maintained in this orientation by a hydrogen bond with Thr-106. A secondary shell made of Trp-116 and Phe-112 fills the space between helices α3 and α6 in hTMPK. Asn-65 is rotated through 120° in Vacc-TMPK compared to the human His-69 and is bound to Lys-105. Together with the lower steric hindrance of the secondary shell residues Ala-107 and Leu-111, it creates a cavity [semitransparent gray volume calculated by using CAVER (52)] between helices α3 and α6 connecting the base binding site to the protein surface.