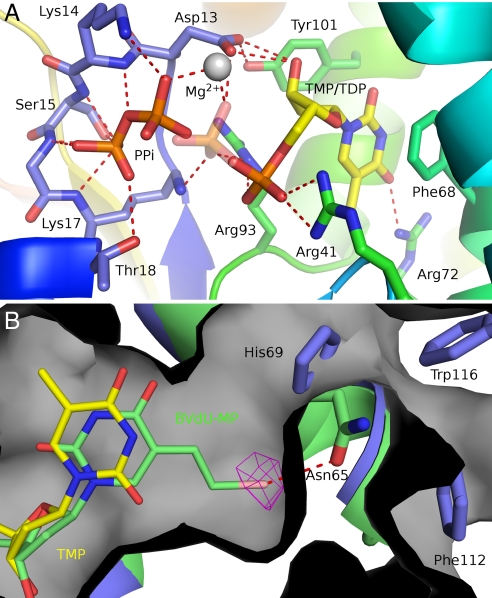
Fig. 3.
The Vacc-TMP kinase active site. (A) View of the active site with the two alternated conformations of the TDP-PPi ligands. The enzyme is rainbow colored, and TMP and PPi are shown as sticks and colored by atom type. Polar interactions are indicated by red dashes. The transferred phosphoryl group here shown at the donor site (PPi) has an alternate conformation (semitransparent phosphate) bound to the thymidine nucleotide. (B) The superimposed crystallographic structures of BVdU-MP (green sticks) and TDP (yellow sticks) at the active site of Vacc-TMPK (green). The bromovinyl group fits into a cavity (gray surface) at the rear of the base binding pocket and is specifically recognized by a halogen bond to Asn-65 (red dashes). The bromine atom is shown in its anomalous difference electron density map contoured at 5 σ. His-69, Trp-116, and Phe-112 close this cavity in the human enzyme (blue).