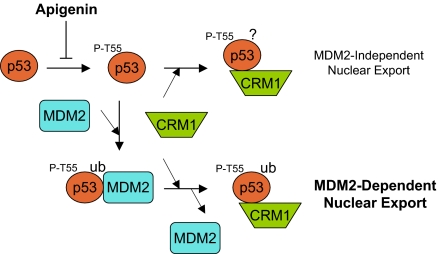
Fig. 5.
A proposed model for the regulation of p53 nuclear export by Thr-55 phosphorylation. In the absence of MDM2, Thr-55 phosphorylation enhances the p53-CRM1 interaction, which leads to nuclear export of p53 in MDM2-null cells. In the presence of MDM2, however, Thr-55 phosphorylation affects the p53-MDM2 interaction, resulting in monoubiquitination of p53, which unmasks p53 NES and promotes CRM1 binding.