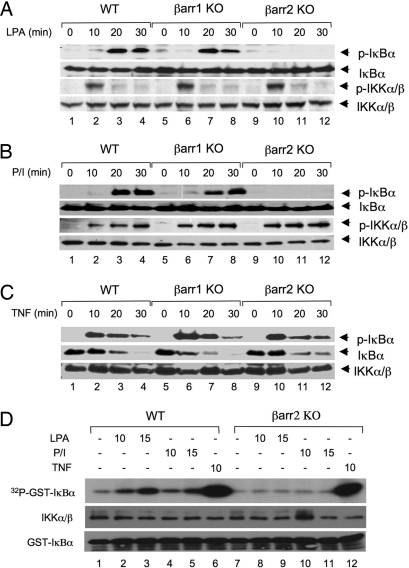
Fig. 2.
LPA- and PMA/Iono-induced IκBα phosphorylation and IKK kinase activity are dependent on β-arrestin 2. (A–C) Wild-type, βarr1 KO, and βarr2 KO MEF cells were stimulated with LPA (10 μM) (A), PMA (40 ng/ml) plus Ionomycin (100 ng/ml) (B), or TNFα (10 ng/ml) (C) for indicated time points. Phosphorylation of IκBα and/or IKK was examined by Western blotting with the indicated antibodies. (D) Wild-type or βarr2 KO MEF cells were stimulated with or without LPA (10 μM), PMA (40 ng/ml) plus Ionomycin (100 ng/ml) (P/I), or TNFα (10 ng/ml) for the indicated time points. The IKK complex was immunoprecipitated by using a mixture of IKKα and IKKγ antibodies and protein A–agarose. The immunoprecipitated complex was subjected to an in vitro kinase assay with GST-IκBα (1–62) as substrates. Parts of the lysates of the immunoprecipitated IKK complex and GST-IκBα (1–62) substrates were subjected to Western blotting as loading controls.