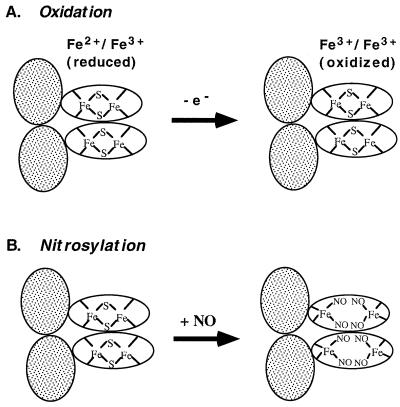
Figure 5.
Distinct activation mechanisms for SoxR. Shaded ovals: DNA binding domains; unshaded ovals, iron-binding domains. SoxR is shown as a homodimer (16). (A) Redox-regulation. When the [2Fe-2S] centers of SoxR are in the reduced state, the protein does not activate soxS transcription. One-electron oxidation converts the protein to a potent transcription activator. (B) Model for activation of SoxR by nitrosylation. The intact [2Fe-2S] clusters of SoxR are directly modified by NO to generate dinitrosyl-iron-dithiol clusters. This modification disrupts the iron-sulfur clusters; the remaining thiols are provided by the cysteine residues of SoxR protein. The model shows all iron atoms remaining bound to the protein, but may not be the case, particularly in the protein subjected to repurification.